

Chinese Journal of Organic Chemistry >
Synthesis of Benzofuran Derivatives by Diphenylperhydroindolinol Silyl Ether-Catalyzed Asymmetric [3+3] Aza-cyclization ofα,β-Unsaturated Aldehydes
Received date: 2020-06-19
Revised date: 2020-08-09
Online published: 2020-08-27
Supported by
the National Natural Science Foundation of China(21962004); the National Natural Science Foundation of China(21562004); the Jiangxi Provincial Department of Science and Technology(20192BAB203004); the Foundation of Jiangxi Provincial Department of Education(GJJ190829); Gannan Medical University(QD201832); Gannan Medical University(QD201816); Gannan Medical University(YJ202027)
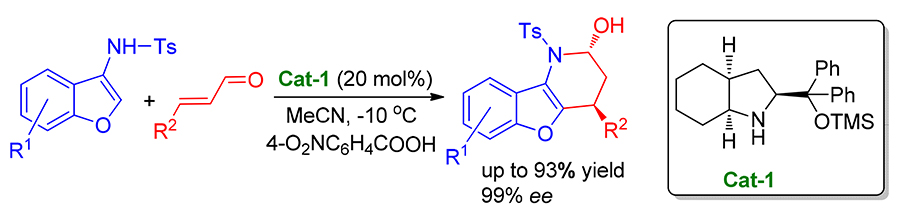
A highly enantioselective [3+3] aza-cyclization of α, β-unsaturated aldehydes with 3-aminobenzofuran promoted by diphenylperhydroindolinol silyl ether has been described, which afforded benzofuran derivatives in high yields (up to 93%), diastereoselectivities ( dr>20∶1) and enantioselectivities (86%~>99% ee). This method also enabled to obtain benzofuran derivatives in gram scale-up.
Huiling Wen , Nianhua Luo , Lu Ouyang , Renshi Luo . Synthesis of Benzofuran Derivatives by Diphenylperhydroindolinol Silyl Ether-Catalyzed Asymmetric [3+3] Aza-cyclization ofα,β-Unsaturated Aldehydes[J]. Chinese Journal of Organic Chemistry, 2021 , 41(1) : 348 -356 . DOI: 10.6023/cjoc202006036
| [1] | (a) Jos M.C.; Maria C.C.; Carolina R.; Mercedes V.; Antonio G.G. Chem. Rev. 2006, 106, 116. |
| [1] | (b) Seephonkai P.; Popescu R.; Zehl M.; Krupitza G.; Urban E.; Kopp B. J. Nat. Prod. 2011, 74, 712. |
| [1] | (c) Liu Y.; Kubo M.; Fukuyama Y. J. Nat. Prod. 2012, 75, 2152. |
| [1] | (d) Rey A.; Couturier C.; Bauer A.; Bronstrup M. Chem. -Eur. J. 2012, 18, 16123. |
| [1] | (e) Liu M.-L.; Duan Y.-H.; Hou Y.-L.; Li C.; Gao H.; Dai Y.; Yao X.-S. Org. Lett. 2013, 15, 1000. |
| [1] | (f) Wang Z.-Yu.; Wong W.-T.; Yang D. Org. Lett. 2013, 15, 4980. |
| [1] | (g) Liu X.; Tian C.; Jiao X.; Li X.; Yang H.; Yao Y. Xie P. Org. Biomol. Chem., 2016, 14, 7715. |
| [1] | (h) Srinivas K.; Ramana C.V. Org. Lett. 2017, 19, 6466. |
| [1] | (i) Smith D.T.; Vitaku E.; Njardarson J.T. Org. Lett. 2017, 19, 3508. |
| [1] | (j) Shrestha A.; Jo H.; Kwon Y.; Lee E.-S. Bioorg. Med. Chem. Lett. 2018, 28, 566. |
| [2] | Savile C.K.; Janey J.M.; Mundorff E.C.; Moore J.C.; Tam S.; Jarvis W.R.; Colbeck J.C.; Krebber A.; Fleitz F.J.; Brands J.; Devine P.N.; Huisman G.W.; Hughes G.J. Science 2010, 329, 305. |
| [3] | Li D.; Wang L.; Yang D.; Zhang B.; Wang R. ACS Catal. 2015, 5, 7432. |
| [4] | (a) Ren H.; Wang P.; Wang L.; Tang Y. Org. Lett. 2015, 17, 4886. |
| [4] | (b) Wang H.; Wang Y.; Zhang C.; Jiang Y.; Chu M.; Li Z.; Du X.; Xu D. Org. Biomol. Chem. 2017, 15, 4191. |
| [5] | Li Z.; Shi Y. Org. Lett. 2015, 17, 5752. |
| [6] | Tian Q.; Bai J.; Chen B.; Zhang G.; Org. Lett. 2016, 18, 1828. |
| [7] | Bos M.; Riguet E. Chem. Commun. 2017, 53, 4997. |
| [8] | Zhang H.; Li S.; Kang Q.; Du Y. Org. Chem. Front. 2019, 6, 3683. |
| [9] | Zhao J.-Q.; Yang L.; Zhou X.-J.; You Y.; Wang Z.-H.; Zhou M.-Q.; Zhang X.-M.; Xu X.-Y.; Yuan W.-C.; Org. Lett. 2019, 21, 660. |
| [10] | Xiao B.-X.; Yan R.-J.; Gao X.-Y.; Du W.; Chen Y.-C. Org. Lett. 2017, 19, 4652. |
| [11] | Şahin E. Chirality 2019, 31, 892. |
| [12] | Rafinski Z. Catalysts 2019, 9, 192. |
| [13] | (a) Wu X.; Xue L.; Li D.; Jia S.; Ao J.; Deng J.; Yan H. Angew. Chem. Int. Ed. 2017, 56, 13722. |
| [13] | (b) Li Y.; Chen K.; Zhang Y.; Sun D.; Ye S. Chin. Chem. Lett. 2018, 29, 1209. |
| [13] | (c) Li X.; Yan J.; Qin J.; Lin S.; Chen W.; Zhan R.; Huang H. J. Org. Chem. 2019, 84, 8035. |
| [14] | Wang C.-S.; Li T.-Z.; Cheng Y.-C.; Zhou J.; Mei G.-J.; Shi F. J. Org. Chem. 2019, 84, 3214. |
| [15] | (a) Ding X.-F.; Su R.-H.; Yang W.-L.; Deng W.-P. Adv. Synth. Catal. 2018, 360, 4168. |
| [15] | (b) Xiao Y.; Wang Y.; Zhou Z. Chin. J. Org. Chem. 2 2019, 39, 2203. (in Chinese) |
| [15] | ( 肖园园, 王有名, 周正洪, 有机化学, 2019, 39, 2203.). |
| [16] | (a) Cheng Q.; Zhang H.-J.; Yue W.-J.; You S.-L. Chem 2017, 3, 428. |
| [16] | (b) Liu L.; Lei L.-S.; Zhan Z.-S.; Liu S.-Z.; Wang Y.-X.; Tu Y.-Q.; Zhang F.-M.; Zhang X.-M.; Ma A.-J.; Wang S.-H. Chem. Commun. 2019, 55, 3789. |
| [17] | (a) Westermann B.; Neuhaus C. Angew. Chem. Int. Ed. 2005, 44, 4077. |
| [17] | (b) Palomo C.; Vera S.; Velilla I.; Mielgo A.; Gomez-Bengoa E. Angew. Chem. Int. Ed. 2007, 46, 8054. |
| [17] | (c) Wang Y.; Shen Z.; Li B.; Zhang Y.; Zhang Y. Chem. Commun. 2007, 1284. |
| [17] | (d) Cao C.-L.; Sun X.-L.; Kang Y.-B.; Tang Y. Org. Lett. 2007, 9, 4151. |
| [17] | (e) Cao C.-L.; Sun X.-L.; Zhou J.-L.; Tang Y. J. Org. Chem. 2007, 72, 4073. |
| [17] | (f) Xie H.; Zu L.; Li H.; Wang J.; Wang W. J. Am. Chem. Soc. 2007, 129, 10886. |
| [17] | (g) Xu L.-W.; Li L.; Shi Z.-H. Adv. Synth. Catal. 2010, 352, 243. |
| [17] | (h) Shi Z.-H.; Sheng H.; Yang K.-F.; Jiang J.-X.; Lai G.-Q.; Lu Y.; Xu L.-W. Eur. J. Org. Chem. 2011, 2011, 66. |
| [17] | (i) Shen H.; Yang K.-F.; Shi Z.-H.; Jiang J.-X.; Lai G.-Q.; Xu L.-W. Eur. J. Org. Chem. 2011, 2011, 5031. |
| [18] | (a) Luo R.-S.; Weng J.; Ai H.-B.; Lu G.; Chan A.S.C. Adv. Synth. Catal. 2009, 351, 2449. |
| [18] | (b) Hu X.; Wei Y.-F.; Wu N.; Jiang Z.; Liu C.; Luo R.-S. Tetrahedron: Asymmetry 2016, 27, 420. |
/
| 〈 |
|
〉 |