

Chinese Journal of Organic Chemistry >
Recent Advances in C—H Fluorination and Amination with N-Fluorobenzenesulfonimide
Received date: 2020-06-29
Revised date: 2020-08-11
Online published: 2020-09-09
Supported by
the Ningbo Municipal Natural Science Foundation(2019A610027); and the Education Foundation of Shaanxi Province(18JK0105)
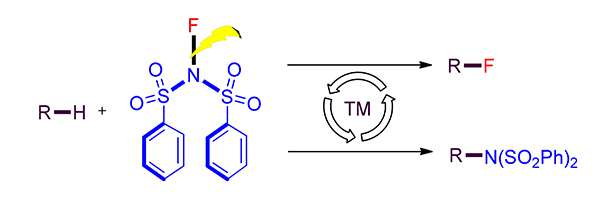
The nitrogen- and fluorine-containing molecules display multiple important bioactivities which are crucial compounds in medicinal chemistry. The strategy relies on the transition-metal-catalyzed C—H amination and fluorination has received much attention due to its atom- and step-economy, providing an alternative to the synthesis of many natural alkaloids and fluorides.N-Fluorobenzenesulfonimide (NFSI) consists of the fluoride atom and the nitrogen-containing functionality, it is frequently used in the reactions based on transition-metal-catalyzed C—H activation to construct both C—N and C—F bonds. In this mini-review, the recent research advances in the formation of C—N and C—F bonds through transition-metal-catalyzed C—H with NSFI are reviewed. The reaction scopes and mechanisms are discussed in details, and the limitations of current procedures and the prospects for the future are summarized.
Weilin Wang , Weidong Chen , Junfei Luo , Pan Xie . Recent Advances in C—H Fluorination and Amination with N-Fluorobenzenesulfonimide[J]. Chinese Journal of Organic Chemistry, 2021 , 41(2) : 543 -552 . DOI: 10.6023/cjoc202006069
| [1] | (a) Liao G.; Wu Y.-J.; Shi B.-F. Acta Chim. Sinica 2020, 78, 289. (in Chinese) |
| [1] | 廖港, 吴勇杰, 史炳锋, 化学学报, 2020, 78, 289.). |
| [1] | (b) Zhao Q.; Meng G.; Nolan S.P.; Szostak M. Chem. Rev. 2020, 120, 1981. |
| [1] | (c) Mao P.; Zhu J.; Yuan J.; Yang L.; Xiao Y.; Zhang C. Chin. J. Org. Chem. 2019, 39, 1529. (in Chinese) |
| [1] | 毛璞, 朱军亮, 袁金伟, 杨亮茹, 肖咏梅, 张长森, 有机化学, 2019, 39, 1529.). |
| [1] | (d) Rej S.; Chatani N. Angew. Chem. Int. Ed. 2019, 58, 8304. |
| [1] | (e) Duarah G.; Kaishap P.P.; Begum T.; Gogoi S. Adv. Synth. Catal. 2019, 361, 654. |
| [1] | (f) Luo F. Chin. J. Org. Chem. 2019, 39, 3084. (in Chinese) |
| [1] | 罗飞华, 有机化学, 2019, 39, 3084.). |
| [1] | (g) Chu J. C. K.; Rovis T. Angew. Chem. Int. Ed. 2018, 57, 62. |
| [1] | (h) Wang S.; Yan F.; Wang L.; Zhu L. Chin. J. Org. Chem. 2018, 38, 291. (in Chinese) |
| [1] | 汪珊, 严沣, 汪连生, 朱磊, 有机化学, 2018, 38, 291.). |
| [2] | (a) Friis S.D.; Johansson M.J.; Ackermann L. Nat. Chem. 2020, 12, 511. |
| [2] | (b) Gruss H.; Sewald N. Chem.- Eur. J. 2020, 26, 5328. |
| [2] | (c) Zhang Y.; Shen S.; Fang H.; Xu T. Org. Lett. 2020, 22, 1244. |
| [2] | (d) Ren Q.; Nie B.; Zhang Y.; Zhang J. Chin. J. Org. Chem. 2018, 38, 2465. (in Chinese) |
| [2] | 任青云, 聂飚, 张英俊, 张霁, 有机化学, 2018, 38, 2465.). |
| [3] | (a) Felpin F.-X.; Sengupta S. Chem. Soc. Rev. 2019, 48, 1150. |
| [3] | (b) Xu P.; Duan X. Chin. J. Org. Chem. 2019, 39, 3315. (in Chinese) |
| [3] | 徐鹏, 段新红, 有机化学, 2019, 39, 3315.). |
| [3] | (c) Nareddy P.; Jordan F.; Szostak M. ACS Cat. 2017, 7, 5721. |
| [4] | (a) Das R.; Kapur M. Asian J. Org. Chem. 2018, 7, 1524. |
| [4] | (b) Luo J.; Xu X.; Zhao Y.; Liang H. Chin. J. Org. Chem. 2017, 37, 2873. (in Chinese) |
| [4] | 骆钧飞, 徐星, 赵延超, 梁洪泽, 有机化学, 2017, 37, 2873.). |
| [4] | (c) Liao G.; Shi B.-F. Acta Chim. Sinica 2015, 73, 1283. (in Chinese) |
| [4] | 廖港, 史炳锋, 化学学报, 2015, 73, 1283.). |
| [5] | (a) Szpera R.; Moseley D. F. J.; Smith L.B.; Sterling A.J. Gouverneur V. Angew. Chem. Int. Ed. 2019, 58, 14824. |
| [5] | (b) He J.; Lou S.; Xu D. Chin. J. Org. Chem. 2016, 36, 1218. (in Chinese) |
| [5] | 何将旗, 娄绍杰, 许丹倩, 有机化学, 2016, 36, 1218.). |
| [5] | (c) Liang T.; Neumann C.N.; Ritter T. Angew. Chem. Int. Ed. 2013, 52, 8214. |
| [6] | (a) Engle K.M.; Mei T.-S.; Wang X.; Yu J.-Q. Angew. Chem. Int. Ed. 2011, 50, 1478. |
| [6] | (b) Wang X.; Leow D.; Yu J.-Q. J. Am. Chem. Soc. 2011, 133, 13864. |
| [6] | (c) Ball N.D.; Gary J.B.; Ye Y.; Sanford M.S. J. Am. Chem. Soc. 2011, 133, 7577. |
| [7] | (a) Rueda-Becerril M.; Sazepin C.C.; Leung J. C. T.; Okbinoglu T.; Kennepohl P.; Paquin J.-F.; Sammis G.M. J. Am. Chem. Soc. 2012, 134, 4026. |
| [7] | (b) Halperin S.D.; Fan H.; Chang S.; Martin R.E.; Britton R. Angew. Chem. Int. Ed. 2014, 53, 4690. |
| [7] | (c) Sibi M.P.; Landais Y. Angew. Chem. Int. Ed. 2013, 52, 3570. |
| [8] | (a) Sibbald P.A.; Rosewall C.F.; Swartz R.D.; Michael F.E. J. Am. Chem. Soc. 2009, 131, 15945. |
| [8] | (b) Weng S.-S.; Hsieh K.-Y.; Zeng Z.-J.; Zhang J.-W. Tetrahedron Lett. 2017, 58, 670. |
| [9] | (a) Qiu S.; Xu T.; Zhou J.; Guo Y.; Liu G. J. Am. Chem. Soc. 2010, 132, 2856. |
| [9] | (b) Zhang H.; Song Y.; Zhao J.; Zhang J.; Zhang Q. Angew. Chem. Int. Ed. 2014, 53, 11079. |
| [10] | (a) Zhang H.; Pu W.; Xiong T.; Li Y.; Zhou X.; Sun K.; Liu Q.; Zhang Q. Angew. Chem. Int. Ed. 2013, 52, 2529. |
| [10] | (b) Wang D.; Wang F.; Chen P.; Lin Z.; Liu G. Angew. Chem. Int. Ed. 2017, 56, 2054. |
| [11] | (a) Zhang B.; Studer A. Org. Lett. 2014, 16, 1790. |
| [11] | (b) Lei B.; Wang X.; Ma L.; Li Y.; Li Z. Org. Biomol. Chem. 2018, 16, 3109. |
| [12] | (a) Wang D.; Wu L.; Wang F.; Wan X.; Chen P.; Lin Z.; Liu G. J. Am. Chem. Soc. 2017, 139, 6811. |
| [12] | (b) Zheng G.; Sun J.; Liu Y.; Yang S.; Li Y.; Sun H.; Zhang Q. J. Org. Chem. 2017, 82, 12813. |
| [13] | (a) Ni Z.; Zhang Q.; Xiong T.; Zheng Y.; Li Y.; Zhang H.; Zhang J.; Liu Q. Angew. Chem. Int. Ed. 2012, 51, 1244. |
| [13] | (b) Zhang X.; Wu R.; Liu W.; Qian D.-W.; Yang J.; Jiang P.; Zheng Q.-Z. Org. Biomol. Chem. 2016, 14, 4789. |
| [13] | (c) Bao F.; Cao Y.; Liu W.; Zhu J. RSC Adv. 2019, 9, 27892. |
| [14] | Lou S.J.; Xu D.Q.; Xia A.B.; Wang Y.F.; Liu Y.K.; Du X.H.; Xu Z.Y. Chem. Commun. 2013, 49, 6218. |
| [15] | Lou S.J.; Xu D.Q.; Xu Z.Y. Angew. Chem. Int. Ed. 2014, 53, 10330. |
| [16] | Lou S.-J.; Chen Q.; Wang Y.-F.; Xu D.-Q.; Du X.-H.; He J.-Q.; Mao Y. -J. Xu, Z.-Y.ACS Catal. 2015, 5, 2846. |
| [17] | Ning X.-Q.; Lou S.-J.; Mao Y.-J.; Xu Z.-Y.; Xu D.-Q. Org. Lett. 2018, 20, 2445. |
| [18] | Testa C.; Gigot E.; Genc S.; Decreau R.; Roger J.; Hierso J.-C. Angew. Chem. Int. Ed. 2016, 55, 5555. |
| [19] | Testa C.; Roger J.; Scheib S.; Fleurat-Lessard P.; Hierso J.C. Adv. Synth. Catal. 2015, 357, 2913. |
| [20] | Ding Q.P.; Ye C.Q.; Pu S.Z.; Cao B.P. Tetrahedron 2014, 70, 409. |
| [21] | Chen C.; Wang C.; Zhang J.; and Zhao Y. J. Org. Chem. 2015, 80, 942. |
| [22] | Gutierrez D.A.; Lee W. -C. C.; Shen Y.; Li J.J. Tetrahedron Lett. 2016, 57, 5372. |
| [23] | Lee J.B.; Kang M.E.; Kim J.; Lee C.Y.; Kee J.-M.; Park K. Myung J.-U.; Hong S.Y. Chem. Commun.; 2017, 53, 10394. |
| [24] | Zhu Q.; Ji D.; Liang T.; Wang X.; Xu Y. Org. Lett. 2015, 17, 3798. |
| [25] | Mao Y.-J.; Lou S.-J.; Hao H.-Y.; Xu D.-Q. Angew. Chem. Int. Ed. 2018, 57, 14085. |
| [26] | Chen Y.-Q.; Singh S.; Wu Y.; Wang Z.; Hao W.; Verma P.; Qiao J.X.; Sunoj R.B.; Yu J.-Q. J. Am. Chem. Soc. 2020, 142, 9966. |
| [27] | Zhu R.Y.; Tanaka K.; Li G.C.; He J.; Fu H.Y.; Li S.H.; Yu J.-Q. J. Am. Chem. Soc. 2015, 137, 7067. |
| [28] | Zhang Q.; Yin X.S.; Chen K.; Zhang S.Q.; Shi B.-F. J. Am. Chem. Soc. 2015, 137, 8219. |
| [29] | Miao J.; Yang K.; Kurek M.; Ge H. Org. Lett. 2015, 17, 3738. |
| [30] | Park H.; Verma P.; Hong K.; Yu J.-Q. Nat. Chem. 2018, 10, 755. |
| [31] | McMurtrey K.B.; Racowski J.M.; Sanford M.S. Org. Lett. 2012, 14, 4094. |
| [32] | (a) Lu S.; Tian L.-L.; Cui T.-W.; Zhu Y.-S.; Zhu X.; Hao X.-Q.; Song M.-P. J. Org. Chem. 2018, 83, 13991. |
| [32] | (b) Haines B.E.; Kawakami T.; Kuwata K.; Murakami K.; Itami K.; Musaev D.G. Chem. Sci. 2017, 8. 988. |
| [32] | (c) Yin Y.; Xie J.; Huang F.-Q.; Qi L.-W.; Zhang B. Adv. Synth. Catal. 2017, 359, 1037. |
| [32] | (d) Barve B.D.; Wu Y.-C.; El-Shazly M.; Korinek M.; Cheng Y.-B.; Wang J.-J.; Chang F.-R. Tetrahedron. 2015, 71, 2290. |
| [32] | (e) Kawakami T.; Murakami K.; Itami K. J. Am. Chem. Soc. 2015, 137, 2460. |
| [32] | (f) Wang S.; Ni Z.; Huang X.; Wang J.; Pan Y. Org. Lett. 2014, 16, 5648. |
| [32] | (g) Wang X.; Lei B.; Ma L.; Jiao H.; Xing W.; Chen J.; Li Z. Adv. Synth. Catal. 2013, 359, 4284. |
| [33] | Tang R.-J.; Luo C.-P.; Yang L.; Li C.-J. Adv. Synth. Catal. 2013, 355, 869. |
| [34] | Dhiman A.K.; Gupta S.S.; Sharma R.; Kumar R.; Sharma U. J. Org. Chem. 2019, 84, 12871. |
| [35] | Iglesias A.; Alvarez R.; de Lera A.R.; Muniz K. Angew. Chem. Int. Ed. 2012, 51, 2225. |
| [36] | Jin L.; Zeng X.; Li S.; Hong X.; Qiu G. Liu. P.Chem. Commun. 2017, 53, 3986. |
| [37] | Dong Y.; Liu G. J. Org. Chem. 2017, 82, 3864. |
| [38] | Sun K.; Li Y.; Xiong T.; Zhang J.; Zhang Q. J. Am. Chem. Soc. 2011, 133, 1694. |
| [39] | Zhou Y.-F.; Zhu J.-M.; Li B.; Zhang Y.; Feng J.; Hall A.; Shi J.-Y.; Zhu W.-L. Org. Lett. 2016, 18, 3803. |
| [40] | Zheng Y.; Xiong T.; Lv Y.; Zhang J.; Zhang Q. Org. Biomol. Chem. 2013, 11, 7923. |
/
| 〈 |
|
〉 |