

Chinese Journal of Organic Chemistry >
Ruthenium-Catalyzed Oxygenative Transformation of Terminal Propargyl Alcohols to Metheyleneketenes via Allenylidene Intermedia-tes: Synthesis ofα,β-Unsaturated Carboxylic Acid Derivatives
Received date: 2020-08-24
Revised date: 2020-09-07
Online published: 2020-09-09
Supported by
the National Natural Science Foundation of China(21971215)
A ruthenium-catalyzed oxygenative transformation of terminal propargyl alcohols to metheyleneketenes via allenylidene intermediates has been developed for the synthesis of a variety of α, β-unsaturated carboxylic acid derivatives. Mechanistic study experiments disclosed that oxygen transfer from pyridine- N-oxide to ruthenium allenylidene generated from the reaction of catalyst CpRuCl(PPh3)2/NaBPh4 with terminal propargyl alcohol resulted in the formation of reactive methyleneketene intermediate, which was trapped into nucleophilic addition reactions to afford α, β-unsaturated product. This reaction offers an attractive complementary strategy to the traditional approach for the synthesis of this class of unsaturated compounds, but in a distinct mechanism, which provides a novel method for the transformation of propargylic alcohols. The metal allenylidene-to-methyleneketene transformation also represents a new mechanistic modality for metal allenylidene-mediated catalysis.
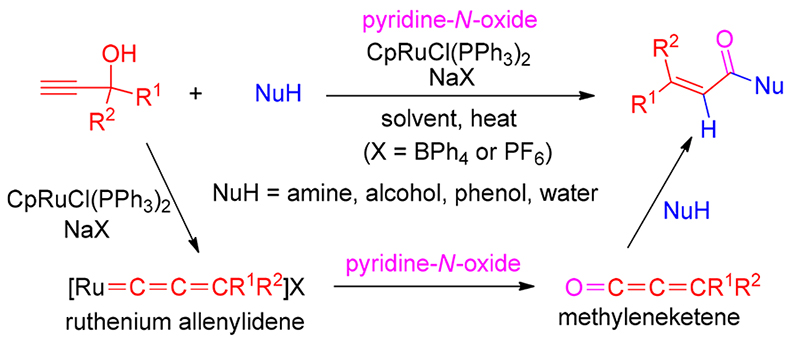
Key words: Keywords ruthenium; allenylidene; propargyl alcohol; oxygen-transfer; methyleneketene; ketene
Xinyu Wang , Qihuan Li , Tingbin Wen . Ruthenium-Catalyzed Oxygenative Transformation of Terminal Propargyl Alcohols to Metheyleneketenes via Allenylidene Intermedia-tes: Synthesis ofα,β-Unsaturated Carboxylic Acid Derivatives[J]. Chinese Journal of Organic Chemistry, 2021 , 41(1) : 284 -296 . DOI: 10.6023/cjoc202008044
| [1] | For selected reviews on the organometallic properties of vinylidene complexes, see: (a) Bruce M. I. Chem. Rev. 1991, 91, 197. |
| [1] | (b) Puerta M.C.; Valerga P. Coord. Chem. Rev. 1999, 193 ~195, 977. |
| [1] | (c) Wakatsuki Y. J. Organomet. Chem. 2004, 689, 4092. |
| [1] | (d) Qiu Z.; Xie Z. Sci. China, Ser. B :Chem. 2009, 52, 1544. |
| [1] | (e) Lynam J.M. Chem. -Eur. J. 2010, 16, 8238. |
| [1] | (f) Herndon J.W. Coord. Chem. Rev. 2018, 356, 1. |
| [2] | For selected reviews on the organometallic properties of allenylidene complexes, see: (a) Bruce M. I. Chem. Rev. 1998, 98, 2797. |
| [2] | (b) Selegue J.P. Coord. Chem. Rev. 2004, 248, 1543. |
| [2] | (c) Rigaut S.; Touchard D.; Dixneuf P.H. Coord. Chem. Rev. 2004, 248, 1585. |
| [2] | (d) Che C.; Ho C.; Huang J. Coord. Chem. Rev. 2007, 251, 2145. |
| [2] | (e) Cadierno V.; Gimeno J. Chem. Rev. 2009, 109, 3512. |
| [2] | (f) Herndon J.W. Coord. Chem. Rev. 2019, 401, 213051. |
| [3] | For selected reviews on metal vinylidenes and allenylidenes in catalysis, see: (a) Bruneau C.; Dixneuf P. H. Acc. Chem. Res. 1999, 32, 311. |
| [3] | (b) Trost B.M.; Toste F.D.; Pinkerton A.B. Chem. Rev. 2001, 101, 2067. |
| [3] | (c) Miki K.; Uemura S.; Ohe K. Chem. Lett. 2005, 34, 1068. |
| [3] | (d) Varela J.A.; Saá C. Chem. -Eur. J. 2006, 12, 6450. |
| [3] | (e) Trost B.M.; McClory A. Chem. -Asian J. 2008, 3, 164. |
| [3] | Bruneau C.; Dixneuf P.H. Metal Vinylidenes and Allenylidenes in Catalysis :From Reactivity to Applications in Synthesis, WILEY-VCH, Weinheim, Germany, 2008. |
| [3] | Varela J.A.; Gonza?lez-Rodríguez C.; Saa? C. Ruthenium in Catalysis, InTopics in Organometallic Chemistry Series 48, Eds.: Bruneau, C.; Dixneuf, P.H., Springer, Switzerland, 2014, pp.237~288. |
| [3] | (h) Roh S.W.; Choi K.; Lee C. Chem. Rev. 2019, 119, 4293. |
| [3] | Gagosz F. Synthesis -Stuttgart 2019, 51, 1087. |
| [3] | (j) Jin J.-T.; Tao X.-C.; Qian Y.-L. Chin. J. Org. Chem. 2000, 20, 470. (in Chinese) |
| [3] | ( 金军挺, 陶晓春, 钱延龙, 有机化学, 2000, 20, 470.). |
| [4] | Coletti C.; Marrone A.; Re N. Acc. Chem. Res. 20 12, 45, 139. |
| [5] | (a) Hyder I.; Jime?nez-Tenorio M.; Puerta M.C.; Valerga P. Organometallics 2011, 30, 726. |
| [5] | (b) Talavera M.; Bolaño S.; Bravo J.; Castro J.; Garcı?a-Fontán S.; Hermida-Ramón J.M. Organometallics 2013, 32, 4402. |
| [5] | (c) Serrano-Ruiz M.; Lidrissi C.; Mañas S.; Peruzzini M.; Romerosa A. J. Organomet. Chem. 2014, 751, 654. |
| [5] | (d) Jiménez-Tenorio M.; Puerta M.C.; Valerga P. Organometallics 2016, 35, 388 and references therein. |
| [6] | (a) Cadierno V.; Gamasa M.P.; Gimeno J.; Perez-Carreno E.; Ienco A. Organometallics 1998, 17, 5216. |
| [6] | (b) Esteruelas M.A.; Gomez A.V.; Lopez A.M.; Onate E.; Ruiz N. Organometallics 1999, 18, 1606. |
| [6] | (c) Cadierno V.; Conejero S.; Gamasa M.P.; Gimeno J.; Falvello L.R.; Llusar R.M. Organometallics 2002, 21, 3716. |
| [6] | (d) Saget T.; Cramer N. Angew. Chem., Int. Ed. 2010, 49, 8962. |
| [6] | (e) Queensen M.J.; Rath N.P.; Bauer E.B. Organometallics 2014, 33, 5052. |
| [6] | (f) García-de la Arada, I.; Díez, J.; Gamasa, M.P.; Lastra, E. J. Organomet. Chem. 2015, 797, 101. |
| [7] | (a) Bustelo E.; Jimenez-Tenorio M.; Puerta M.C.; Valerga P. Organometallics 2006, 25, 4019. |
| [7] | (b) Pino-Chamorro J.A.; Bustelo E.; Puerta M.C.; Valerga P. Organometallics 2009, 28, 1546. |
| [8] | (a) Trost B.M.; Frederiksen M.U.; Rudd M.T. Angew. Chem., Int. Ed. 2005, 44, 6630. |
| [8] | (b) Bruneau C.; Dixneuf P.H. Angew. Chem., Int. Ed. 2006, 45, 2176. |
| [8] | (c) Liu R.-S. Synlett 2008, 801. |
| [9] | (a) Nishibayashi Y.; Uemura S. Curr. Org. Chem 2006, 10, 135. |
| [9] | Nishibayashi Y. Synthesis 2012, 489. |
| [9] | (c) Sakata K.; Nishibayashi Y. Catal. Sci. Technol. 2018, 8, 12. |
| [10] | Zhang D.-Y.; Hu X.-P. Tetrahedron Lett. 2015, 56, 283. |
| [11] | Trost B.M.; Flygare J.A. J. Am. Chem. Soc. 1992, 114, 5476. |
| [12] | (a) Bustelo E.; Dixneuf P.H. Adv. Synth. Catal. 2005, 347, 393. |
| [12] | (b) Ma H.W.; Lin Y.C.; Huang S.L. Org. Lett. 2012, 14, 3846. |
| [13] | (a) Yeh K.L.; Liu B.; Lo C.Y.; Huang H.L.; Liu R.S. J. Am. Chem. Soc. 2002, 124, 6510. |
| [13] | (b) Yeh K.L.; Liu B.; Lai Y.T.; Li C.W.; Liu R.S. J. Org. Chem. 2004, 69, 4692. |
| [13] | (c) Shen H.C.; Su H.L.; Hsueh Y.C.; Liu R.S. Organometallics 2004, 23, 4332. |
| [13] | Propargylic reduction of propargylic alcohols with 2-Propanol via similar hydrogen transfer was reported:. |
| [13] | (d) Yuki M.; Miyake Y.; Nishibayashi Y. Organometallics 2010, 29, 5994. |
| [14] | Datta S.; Chang C.L.; Yeh K.L.; Liu R.S. J. Am. Chem. Soc. 2003, 125, 9294. |
| [15] | (a) Cadierno V.; Díez J.; García-Garrido S.E.; Gimeno J. Chem. Commun. 2004, 2716. |
| [15] | (b) Cadierno V.; Díez J.; García-Garrido S.E.; Gimeno J.; Nebra N. Adv. Synth. Catal. 2006, 348, 2125. |
| [15] | (c) Cadierno V.; García-Garrido S.E.; Gimeno J. Adv. Synth. Catal. 2006, 348, 101. |
| [15] | (d) Onodera G.; Matsumoto H.; Nishibayashi Y.; Uemura Y. Organometallics 2005, 24, 5799. |
| [16] | (a) Tidwell T.T. Angew. Chem., Int. Ed. 2005, 44, 5778. |
| [16] | (b) Allen A.D.; Tidwell T.T. Eur. J. Org. Chem. 2012, 2012, 1081. |
| [16] | (c) Allen A.D.; Tidwell T.T. Chem. Rev. 2013, 113, 7287. |
| [17] | (a) Madhushaw R.J.; Lin M.Y.; Abu Sohel S.M.; Liu R.S. J. Am. Chem. Soc. 2004, 126, 6895. |
| [17] | (b) Lin M.Y.; Madhushaw R.J.; Liu R.S. J. Org. Chem. 2004, 69, 7700. |
| [17] | (c) Lin M.Y.; Maddirala S.J.; Liu R.S. Org. Lett. 2005, 7, 1745. |
| [17] | (d) Pati K.; Liu R.S. Chem. Commun. 2009, 5233. |
| [17] | (e) Kim I.; Lee C. Angew. Chem., Int. Ed. 2013, 52, 10023. |
| [17] | (f) Kim I.; Roh S.W.; Lee D.G.; Lee C. Org. Lett. 2014, 16, 2482. |
| [17] | (g) Wang Y.; Zheng Z.; Zhang L. Angew. Chem., Int. Ed. 2014, 53, 9572. |
| [17] | (h) Zheng R.; Wang Y.; Zhang L. Tetrahedron Lett. 2015, 56, 3144. |
| [17] | (i) Zeng H.; Li C.J. Angew. Chem., Int. Ed. 2014, 53, 13862. |
| [17] | (j) Yu C.; Ma X.; Chen B.; Tang B.; Paton R.S.; Zhang G. Eur. J. Org. Chem. 2017, 2017, 1561. |
| [17] | (k) Rong M.G.; Qin T.Z.; Liu X.R.; Wang H.F.; Zi W. Org. Lett. 2018, 20, 6289. |
| [17] | (l) Zhang W.W.; Gao T.T.; Xu L.J.; Li B.J. Org. Lett. 2018, 20, 6534. |
| [17] | (m) Álvarez-Pérez A.; Esteruelas M.A.; Izquierdo S.; Varela J.A.; Saá C. Org. Lett. 2019, 21, 5346. |
| [17] | For a recent review, see:. |
| [17] | Álvarez-Pérez A.; Varela J.A.; Saá C. Synthesis 2020, 52, 2639. |
| [18] | Brown R.F.C.; Eastwood F.W. The Chemistry of Ketenes, Allenes and Related Compounds, John Wiley& Sons Ltd, New York , 1980, Chapter 19. |
| [19] | (a) Hart H.; Dean D.L.; Buchanan D.N. J. Am. Chem. Soc. 1973, 95, 6294. |
| [19] | (b) Chapman O.L.; Chang C.-C.; Hole J.; Rosenquist N.R.; Tomioka H. J. Am. Chem. Soc. 1975, 97, 22, 6586. |
| [19] | (c) Meng J.B.; Shen M.Q.; Wang X.H.; Gao Z.H.; Wang H.G.; Matsuura T. Chin. Sci. Bull. 1991, 36, 2056. |
| [19] | (d) Pietri N.; Monnier M.; Aycard J.P. J. Org. Chem. 1998, 63, 2462. |
| [19] | (e) Yang C.; Wu W.; Liu K.; Wang H.; Su H. Sci. China :Chem. 2012, 55, 359. |
| [20] | (a) Brown R.F.C.; Jones C.M. Aust. J. Chem. 1980, 33, 1817. |
| [20] | (b) Brown R.F.C.; Eastwood F.W.; Chaichit N.; Gatehouse B.M.; Pfeiffer J.M.; Woodroffe D. Aust. J. Chem. 1981, 34, 1467. |
| [20] | (c) Besida J.; Brown R.F.C. Aust. J. Chem. 1982, 35, 1385. |
| [20] | (d) Besida J.; Brown R.F.C.; Colmanet S.; Leach D.N. Aust. J. Chem. 1982, 35, 1373. |
| [20] | (e) Pommelet J.C.; Dhimane H.; Chuche J.; Celerier J.P.; Haddad M.; Lhommet G. J. Org. Chem. 1988, 53, 5680. |
| [20] | (f) Wentrup C.; Lorencak P. J. Am. Chem. Soc. 1988, 110, 1880. |
| [20] | (g) Brahms J.C.; Dailey W.P. J. Am. Chem. Soc. 1989, 111, 8940. |
| [20] | (h) Bencheikh A.; Pommelet J.C.; Chuche J. J. Chem. Soc., Chem. Commun. 1990, 615. |
| [20] | (i) Chuburu F.; Lacombe S.; Pfisterguillouzo G.; Bencheik A.; Chuche J.; Pommelet J.C. J. Am. Chem. Soc. 1991, 113, 1954. |
| [20] | (j) Fulloon B.E.; Wentrup C. J. Org. Chem. 1996, 61, 1363. |
| [20] | Gaber A.A.M.; McNab H. Synthesis -Stuttgart 2001, 2059. |
| [20] | (l) Halton B.; Dixon G.M.; Jones C.S.; Parkin C.T.; Veedu R.N.; Bornemann H.; Wentrup C. Org. Lett. 2005, 7, 949. |
| [20] | (m) Andersen H.G.; Wentrup C. Aust. J. Chem. 2012, 65 . |
| [21] | (a) Birum G.H.; Matthews C.N. J. Am. Chem. Soc. 1968, 90, 14, 3842. |
| [21] | (b) Taylor G.A. Chem. Commun. (London )1968, 1314. |
| [21] | (c) Taylor G.A. J. Chem. Soc. 1969, 1755. |
| [21] | (c) Masters A.P.; Sorensen T.S.; Tran P.M. Can. J. Chem. 1987, 65, 1499. |
| [22] | Cai T.; Yang Y.; Zhang L.; Wen T. Chin. J. Org. Chem. 2018, 38, 2017. (in Chinese) |
| [22] | ( 蔡涛, 杨玉, 张丽, 温庭斌, 有机化学, 2018, 38, 2017.). |
| [23] | For catalytic nitrogen tranfer to metal vinylidenes with hydrazines for nitrile synthesis, see: (a) Fukumoto Y.; Dohi T.; Masaoka H.; Chatani N.; Murai S. Organometallics 2002, 21, 3845. |
| [23] | (b) Fukumoto Y.; Tamura Y.; Iyori Y.; Chatani N. J. Org. Chem. 2016, 81, 3161. |
| [23] | For stoichiometrc nitrogen tranfer to metal vinylidenes with hydrazines to give nitrile complexes, see: (c) Alt H.G.; Engelhardt H.E.; Steinlein E.; Rogers D. J. Organomet. Chem. 1987, 344, 321. |
| [23] | (d) Barrett A.G.M.; Carpenter N.E.; Sabat M. J. Organomet. Chem. 1988, 352, C8. |
| [23] | (e) Albertin G.; Antoniutti S.; Bortoluzzi M.; Botter A.; Castro J. Dalton Trans. 201 5, 44, 3439. |
| [24] | (a) Arshad L.; Jantan I.; Bukhari S.N.; Haque M.A. Front Pharmacol 2017, 8, 22. |
| [24] | (b) Hossain M.; Das U.; Dimmock J.R. Eur. J. Med. Chem. 2019, 183, 111687. |
| [24] | (c) Zhang S.; Neumann H.; Beller M. Chem. Soc. Rev. 2020, 49, 3187. |
| [25] | (a) Reichl K.D.; Dunn N.L.; Fastuca N.J.; Radosevich A.T. J. Am. Chem. Soc. 2015, 137, 5292. |
| [25] | (b) Meng L.K.; Kamada Y.; Muto K.; Yamaguchi J.; Itami K. Angew. Chem., Int. Ed. 2013, 52, 10048. |
| [25] | (c) Liu L.; Lu H.; Wang H.; Yang C.; Zhang X.; Zhang-Negrerie D.; Du Y.F.; Zhao K. Org. Lett. 2013, 15, 2906. |
| [25] | (d) Li Y.J.; Yang Q.; Yang L.Q.; Lei N.; Zheng K. Chem. Commun. 2019, 55, 4981. |
| [26] | Bruce M.I.; Low P.J.; Tiekink E.R.T. J. Organomet. Chem. 1999, 572, 3. |
| [27] | Following Saá’s conditions for the oxidative amidation of alkynes(see Ref.[17m]),1 equiv. of NaPF6 was used. The PF6 – anion is prone to dissociate into PF5 and F– at elevated temperature and the residual water presented in the solution may cause further hydrolysis of the resulting PF5 species to form phosphate. See: (a) Odedra A.; Datta S.; Liu R. S. J. Org. Chem. 2007, 72, 3289. |
| [27] | (b) Krossing I.; Raabe I. Chem. -Eur. J. 2004, 10, 5017. |
| [27] | (c) Krossing I.; Raabe I. Angew. Chem., Int. Ed. 2004, 43, 2066. |
| [28] | The DCE solvent may undergo dissociation to eliminate hydrogen chloride after prolonged heating. See: (a) Ho, M. L.; Flynn, A. B.; Ogilvie, W. W.J. Org. Chem. 2007, 72, 977. |
| [28] | (b) He W.; Xie L.; Xu Y.; Xiang J.; Zhang L. Org. Biomol. Chem. 2012, 10, 3168. |
| [28] | (c) Qian G.; Hong X.; Liu B.; Mao H.; Xu B. Org. Lett. 2014, 16, 5294. |
| [29] | (a) Pavlik S.; Mereiter K.; Puchberger M.; Kirchner K. J. Organomet. Chem. 2005, 690, 5497. |
| [29] | (b) Hartmann S.; Winter R.F.; Brunner B.M.; Sarkar B.; Knodler A.; Hartenbach I. Eur. J. Inorg. Chem. 2003, 876. |
| [29] | (c) Chan W.C.; Lau C.P.; Chen Y.Z.; Fang Y.Q.; Ng S.M.; Jia G.C. Organometallics 1997, 16, 34. |
| [29] | (d) Buriez B.; Burns I.D.; Hill A.F.; White A.J.P.; Williams D.J.; Wilton-Ely J.D.E. T. Organometallics 1999, 18, 1504. |
| [30] | (a) Picquet M.; Bruneau C.; Dixneuf P.H. Chem. Commun. 1998, 2249. |
| [30] | (b) Furstner A.; Liebl M.; Lehmann C.W.; Picquet M.; Kunz R.; Bruneau C.; Touchard D.; Dixneuf P.H. Chem. -Eur. J. 2000, 6, 1847. |
| [31] | Selegue J.P. J. Am. Chem. Soc. 1983, 105, 5921. |
| [32] | (a) Lukehart C.M.; Zelie J.V. J. Organomet. Chem. 1975, 97, 421. |
| [32] | (b) Wulff W.D.; Yang D.C. J. Am. Chem. Soc. 1983, 105, 6726. |
| [32] | (c) Dötz K.H. Angew. Chem., Int. Ed. 1984, 23, 587. |
| [32] | (d) Barrett A.G.M.; Mortier J.; Sabat M.; Sturgess M.A. Organometallics 1988, 7, 2553. |
| [32] | (e) Gibert M.; Ferrer M.; Lluch A.M.; Sánchez-Baeza F.; Messeguer A. J. Org. Chem. 1999, 64, 1591. |
| [32] | (f) Ruan W.; Shi C.; Sung H.H.Y.; Williams I.D.; Jia G. J. Organomet. Chem. 2019, 880, 7. |
| [33] | (a) Trost B.M.; Rhee Y.H. J. Am. Chem. Soc. 1999, 121, 11680. |
| [33] | (b) Trost B.M.; Rhee Y.H. J. Am. Chem. Soc. 2002, 124, 2528. |
| [33] | (c) Taduri B.P.; Sohel S.M.A.; Cheng H.M.; Lin G.Y.; Liu R.S. Chem. Commun. 2007, 2, 2530. |
| [34] | Bruce M.I.; Hameister C.; Swincer A.G.; Wallis R.C. Inorg. Synth. 1990, 28, 270. |
| [35] | Gluyas J.B.G.; Brown N.J.; Farmer J.D.; Low P.J. Aust. J. Chem. 2017, 70, 113. |
| [36] | Cai T.; Yang Y.; Li W.W.; Qin W.B.; Wen T.B. Chem. -Eur. J. 2018, 24, 1606. |
| [37] | Alcock N.W.; Burns I.D.; Claire K.S.; Hill A.F. Inorg. Chem. 1992, 31, 2906. |
| [38] | Boren B.C.; Narayan S.; Rasmussen L.K.; Zhang L.; Zhao H.T.; Lin Z.Y.; Jia G.C.; Fokin V.V. J. Am. Chem. Soc. 200 8, 130, 14900. |
| [39] | (a) Harada S.; Yano H.; Obora Y. ChemCatChem 2013, 5, 121. |
| [39] | (b) Han Y.P.; Song X.R.; Qiu Y.F.; Hao X.H.; Wang J.; Wu X.X.; Liu X.Y.; Liang Y.M. J. Org. Chem. 2015, 80, 9200. |
| [39] | (c) Groundwater P.W.; Garnett I.; Morton A.J.; Sharif T.; Coles S.J.; Hursthouse M.B.; Nyerges M.; Anderson R.J.; Bendell D.; McKillop A.; Zhang W. J. Chem. Soc., Perkin Trans. 1 2001, 2781. |
| [39] | (d) Ueda S.; Okada T.; Nagasawa H. Chem. Commun. 2010, 46, 2462. |
| [39] | (e) Reeves D.C.; Rodriguez S.; Lee H.; Haddad N.; Krishnamurthy D.; Senanayake C.H. Org. Lett. 2011, 13, 2495. |
| [39] | (f) Song C.E.; Jung D.U.; Choung S.Y.; Roh E.J.; Lee S.G. Angew. Chem., Int. Ed. 2 004, 43, 6183. |
| [39] | (g) Inamoto K.; Okawa H.; Taneda H.; Sato M.; Hirono Y.; Yonemoto M.; Kikkawa S.; Kondo Y. Chem. Commun. 2012, 48, 9771. |
| [39] | (h) Ito Y. Tetrahedron 2007, 63, 3108. |
/
| 〈 |
|
〉 |