

Chinese Journal of Organic Chemistry >
Recent Progress in the Selenocyclization Reactions with Organic Selenides
Received date: 2020-08-08
Revised date: 2020-09-09
Online published: 2020-09-30
Supported by
Outstanding Youth Project of Natural Science Foundation of Heilongjiang Province(YQ2019B004); General Project of Natural Foundation of Heilongjiang Province(H2017001); Youth Innovation Talent Project of Harbin University of Commerce(2016QN056); Youth Innovation Talent Project of Harbin University of Commerce(2019CX38); Youth Reserve Talent Program of Harbin University of Commerce(2019CX36); Natural Foundation Co-pilot Project of Heilongjiang Province(LH2020H068); Natural Foundation Co-pilot Project of Heilongjiang Province(LH2020H070)
Organic selenides are a kind of important molecules, which are widely used in medicine, agricultural chemicals, organic materials and catalysis, the introduction of selenium atom into organic molecules is of great significance in the synthetic chemistry. Heterocyclic compounds are key skeletons involved in a variety of bioactive molecules, therefore, the development of new methods for the synthesis of selenium-containing heterocyclic derivatives has attracted much attentions. The recent progress in this rapidly growing area, including metal catalysis, electrochemical catalysis, visible-light catalysis, organocatalysis, and other selenocyclization types, is highlighted with an emphasis on the scope and the mechanisms of these different reactions.
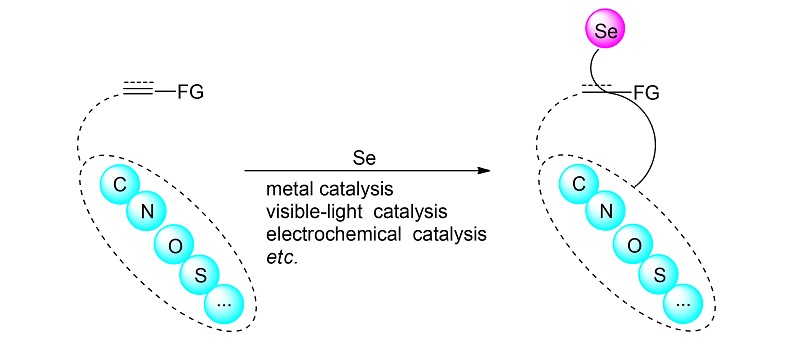
Key words: organic selenide; heterocyclic compound; selenocyclization; catalysis
Ying Xu , Chen Li , Jianping Meng , Yuling Huang , Jiyuan Fu , Bing Liu , Yingjie Liu , Ning Chen . Recent Progress in the Selenocyclization Reactions with Organic Selenides[J]. Chinese Journal of Organic Chemistry, 2021 , 41(3) : 1012 -1030 . DOI: 10.6023/cjoc202008009
| [1] | (a) Zeni, G.; Braga, A. L.; Stefani, H. A. Acc. Chem. Res. 2003, 36, 731. |
| [1] | (b) Peng, X.-L.; Ma, C.; Tung, C.; Xu, Z.-H. Org. Lett. 2016, 18, 4154. |
| [1] | (c) Huang, S.; Li, H.; Xie, T.; Wei, F.; Tung, C.; Xu, Z.-H. Org. Chem. Front. 2019, 6, 1663. |
| [1] | (d) Wang, W.-G.; Huang, S.; Yan, S.-K.; Sun, X.-J.; Tung, C.; Xu, Z.-H. Chin. J. Chem. 2020, 38, 445. |
| [2] | Some examples: (a) Vinogradova, E. V.; Zhang, C.; Spokoyny, A. M.; Pentelute, B. L.; Buchwald, S. L. Nature 2015, 526, 687. |
| [2] | (b) Nogueira, C. W.; Zeni, G.; Rocha, J. B. T. Chem. Rev. 2004, 104, 6255. |
| [2] | (c) Manna, D.; Roy, G.; Mugesh, G. Acc. Chem. Res. 2013, 46, 2706. |
| [2] | (d) Reich, H. J.; Hondal, R. J. ACS Chem. Biol. 2016, 11, 821. |
| [3] | Guan, Q.; Han, C.-M.; Zuo, D.-Y.; Zhai, M.-A.; Li, Z.-Q.; Zhang, Q.; Zhai, Y.-P.; Jiang, X.-W.; Bao, K.; Wu, Y.-L.; Zhang, W.-G. Eur. J. Med. Chem. 2014, 87, 306. |
| [4] | Sajomsang, W.; Tantayanon, S.; Tangpasuthadol, V.; Daly, W. H. Carbohydr. Res. 2009, 344, 2502. |
| [5] | Wen, Y.; Xu, J.-W.; Wang, Z.-W.; Qi, H.; Xu, Q.-L.; Bai, Z.-S.; Zhang, Q.; Bao, K.; Wu, Y.-L.; Zhang, W. G. Eur. J. Med. Chem. 2015, 90, 184. |
| [6] | Kumar, S.; Sharma, N.; Maurya, I. K.; Bhasin, A. K. K.; Wangoo, N.; Brandao, P.; Fleix, V.; Bhasin, K. K.; Kumar, R. K. Eur. J. Med. Chem. 2016, 123, 916. |
| [7] | (a) Brutchey, R. L. Acc. Chem. Res. 2015, 48, 2918. |
| [7] | (b) Patra, A.; Wijsboom, Y. H.; Leitus, G.; Bendikov, M. Chem. Mater. 2011, 23, 896. |
| [8] | (a) Nogueira, C. W.; Rocha, J. B. T. Arch. Toxicol. 2011, 85, 1313. 0db53dfe-c7ef-4148-9991-9a83b2815da0 |
| [8] | (b) Nogueira, C. W.; Zeni, G.; Rocha, J. B. T. Chem. Rev. 2004, 104, 6255. |
| [9] | Some examples: (a) Nunes, V. L.; de Oliveira, I. C.; Barros, O. S. D. Eur. J. Org. Chem. 2014, 2014, 1525. |
| [9] | (b) Santi, C.; Santoro, S.; Testaferri, L.; Tiecco, M. Synlett 2008,1471. |
| [9] | (c) Santi, C.; Santoro, S. In Organoselenium Chemistry: Synthesis and Reactions, Ed.: Wirth, T., Wiley-VCH, Germany, 2011, pp.1~51. |
| [9] | (d) Ogawa, A.; Ogawa, I.; Obayashi, R.; Umezu, K.; Doi, M.; Hirao, T. J. Org. Chem. 1999, 64, 86. |
| [10] | Some examples: (a) Perin, G.; Lenarda?o, E. J.; Jacob, R. G.; Panatieri, R. B. Chem. Rev. 2009, 109, 1277. |
| [10] | (b) Sun, K.; Shi, Z.; Liu, Z.; Luan, B.; Zhu, J.; Xue, Y. Org. Lett. 2018, 20, 6687. |
| [10] | (c) Sun, K.; Wang, X.; Lv, Y.; Li, G.; Jiao, H.; Dai, C.; Li, Y.; Zhang, C.; Liu, L. Chem. Commun. 2016, 52, 8471. |
| [10] | (d) Sun, K.; Wang, X.; Fu, F.; Zhang, C.; Chen, Y.; Liu, L. Green Chem. 2017, 19, 1490. |
| [10] | (e) Wang, X.; Mu, S.-Q.; Sun, T.; Sun, K. Chin. J. Org. Chem. 2019, 39, 2802. (in Chinese) |
| [10] | (王薪, 穆石强, 孙婷, 孙凯, 有机化学, 2019, 39, 2802.) |
| [10] | (f) Sun, K.; Wang, X.; Li, C.; Wang, H.; Li, L. Org. Chem. Front. 2020, 7, 3100. |
| [11] | Some examples: (a) Bax, B. D.; Chan, P. F.; Eggleston, D. S.; Fosberry, A.; Gentry, D. R.; Gorrec, F.; Giordano, I.; Hann, M. M.; Hennessy, A.; Hibbs, M.; Huang, J.; Jones, E.; Jones, J.; Brown, K. K.; Lewis, C. J.; May, E. W.; Saunders, M. R.; Singh, O.; Spitzfaden, C. E.; Shen, C.; Shillings, A.; Theobald, A. J.; Wohlkonig, A.; Pearson, N. D.; Gwynn, M. N. Nature 2010, 466, 935. |
| [11] | (b) Rouffet, M.; de Oliveira, C. A. F.; Udi, Y.; Agrawal, A.; Sagi, I.; McCammon, J. A.; Cohen, S. M. J. Am. Chem. Soc. 2010, 132, 8232. |
| [11] | (c) Andrews, S.; Burgess, S. J.; Skaalrud, D.; Kelly, J. X.; Peyton, D. H. J. Med. Chem. 2010, 53, 916. |
| [11] | (d) Sun, K.; Li, Y.; Feng, R.; Mu, S.; Wang, X.; Zhang, B. J. Org. Chem. 2020, 85, 1001. |
| [11] | (e) Sun, K.; Li, G.; Li, Y.; Yu, J.; Zhao, Q.; Zhang, Z.; Zhang, G. Adv. Synth. Catal. 2020, 362, 1947. |
| [11] | (f) Wang, X.; Li, G.; Sun, K.; Zhang, B. Chin. J. Org. Chem. 2020, 40, 913. (in Chinese) |
| [11] | (王薪, 李国锋, 孙凯, 张冰, 有机化学, 2020, 40, 913.) |
| [11] | (g) Sun, K.; Wang, X.; Wang, Q.; Xue, Y.; Wu, L.; Zhang, B. Chem. Commun. 2020, 56, 4436. |
| [11] | (h) Meng, X.; Kang, Q.; Zhang, J.; Li, Q.; Wei, W.; He, W. Green Chem. 2020, 22, 1388. |
| [11] | (i) Kang, Q.; Wu, W.; Li, Q.; Wei, W. Green Chem. 2020, 22, 3060. |
| [12] | Some examples: (a) Cherney, A. H.; Kadunce, N. T.; Reisman, S. E. Chem. Rev. 2015, 115, 9587. |
| [12] | (b) Finkbeiner, P.; Kloeckner, U.; Nachtsheim, B. J. Angew. Chem., Int. Ed. 2015, 54, 4949. |
| [12] | (c) Riedmuller, S.; Kaulfhold, O.; Spreitzer, H.; Nachtsheim, B. J. Eur. J. Org. Chem. 2014, 2014, 1391. |
| [12] | (d) Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780. |
| [13] | Some examples: (a) Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V. J. Organomet. Chem. 2000, 605, 96. |
| [13] | (b) Beletskaya, I. P.; Ananikov, V. P. Pure Appl. Chem. 2007, 79, 1041. |
| [14] | Stein, A. L.; Alves, D.; da Rocha, J. T.; Nogueira, C. W.; Zeni, G. Org. Lett. 2008, 10, 4983. |
| [15] | Du, H.-A.; Zhang, X.-G.; Tang, R.-Y.; Li, J.-H. J. Org. Chem. 2009, 74, 7844. |
| [16] | Gay, R. M.; Manarin, F.; Schneider, C. C.; Barancelli, D. A.; Costa, M. D.; Zeni, G. J. Org. Chem. 2010, 75, 5701. |
| [17] | Li, Z.; Hong, L.; Liu, R.; Shen, J.; Zhou, X. Tetrahedron 2011, 52, 1343. |
| [18] | Schumacher, R. F.; Rosario, A. R.; Leite, M. R.; Zeni, G. ChemInform 2013, 19, 13059. |
| [19] | Mantovani, A. C.; Goulart, T. A. C.; Back, D. F.; Menezes, P. H.; Zeni, G. J. Org. Chem. 2014, 79, 10526. |
| [20] | Maity, P.; Kundu, D.; Roy, R.; Ranu, B. C. Org. Lett. 2014, 16, 4122. |
| [21] | Reddy, A. S.; Swamy, K. C. Org. Lett. 2015, 17, 2996. |
| [22] | Stein, A. L.; Rosario, A. R.; Zeni, G. Eur. J. Org. Chem. 2015, 2015, 5640. |
| [23] | Wang, W.; Peng, X.; Wei, F.; Tung, C.; Xu, Z. Angew. Chem., Int. Ed. 2016, 55, 649. |
| [24] | Yamada, M.; Matsumura, M.; Takino, F.; Murata, Y.; Kurata, Y.; Kawahata, M.; Yamaguchi, K.; Kakusawa, N.; Yasuike, S. Eur. J. Org. Chem. 2018, 2018, 170. |
| [25] | Some examples: (a) Yamada, M.; Matsumura, M.; Uchida, Y.; Kawahata, M.; Murata, Y.; Kaku-sawa, N.; Yamaguchi, K.; Yasuike, S. Beilstein J. Org. Chem. 2016, 12, 13093. |
| [25] | (b) Yamada, M.; Matsumura, M.; Murata, Y.; Kawahata, M.; Saito, K.; Kakusawa, N.; Yamaguchi, K.; Yasuike, S. Tetrahedron 2017, 73, 2614. |
| [26] | Some examples: (a) Ricordi, V. G.; Thurow, S.; Penteado, F.; Schumacher, R. F.; Perin, G.; Lenard?o, E. J.; Alves, D. Adv. Synth. Catal. 2015, 357, 933. |
| [26] | (b) Stein, A. L.; Bilheri, F. N.; Rocha, J. T.; Back, D. F.; Zeni, G. Chem.-Eur. J. 2012, 18, 10602. 1a0de804-d1dc-4424-806b-78c5167e81e6 |
| [26] | (c) Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V.; Khrustalev, V. N. Chem. Lett. 2010, 39, 720. |
| [27] | Goulart, T. A. C.; Back, D. F.; Zeni, G. Adv. Synth. Catal. 2017, 359, 1901. |
| [28] | Kaz-mierczak, J. C.; Recchi, A. M. S.; Gritzenco, F.; Balbom, E. B.; Barcellos, T.; Speranc?a, A.; Godoi, B. Eur. J. Org. Chem. 2017, 2017, 6382. |
| [29] | Casola, K. K.; Gomes, M. R.; Back, D. F.; Zeni, G. J. Org. Chem. 2018, 83, 6706. |
| [30] | Cui, F.; Chen, J.; Mo, Z.; Su, S.; Chen, Y.; Ma, X.; Tang, H.; Wang, H.; Pan, Y.; Xu, Y.-L. Org. Lett. 2018, 20, 925. |
| [31] | An, C.; Li, C.-Y.; Huang, X.-B.; Gao, W.-X.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Org. Lett. 2019, 21, 6710. |
| [32] | Some examples: (a) Yan, J.; Xu, J.; Zhou, Y.; Chen, J.; Song, Q. Org. Chem. Front. 2018, 5, 1483. |
| [32] | (b) Xu, J.; Yu, X.; Yan, J.; Song, Q. Org. Lett. 2017, 19, 6292. |
| [32] | (c) Liu, W.; Hu, Y.-Q.; Hong, X.-Y.; Li, G.-X.; Huang, X.-B.; Gao, W.-X.; Liu, M.-C.; Xia, Y.-Z.; Zhou, Y.-B.; Wu, H.-Y. Chem. Commun. 2018, 54, 14148. |
| [33] | Sun, K.; Wang, S.; Feng, R.; Zhang, Y.; Wang, X.; Zhang, Z.; Zhang, B. Org. Lett. 2019, 21, 2052. |
| [34] | Ren, P.-X.; Qi, L.; Fang, Z.-Y.; Wu, T.-S.; Gao, Y.-M.; Shen, S.; Song, J.-Y.; Wang, L.-J.; Li, W. Chin. J. Org. Chem. 2019, 39, 1776. (in Chinese) |
| [34] | (任培星, 齐林, 方卓越, 吴天舒, 高雅蒙, 沈松, 宋金燕, 王力竞, 李玮, 有机化学, 2019, 39, 1776.) |
| [35] | Zhou, X.-G.; Wang, S.-K.; Li, Z.; Liu, R.-T. Chin. J. Org. Chem. 2019, 39, 3215. (in Chinese) |
| [35] | (周锡庚, 王圣克, 李振, 刘瑞婷, 有机化学, 2019, 39, 3215.) |
| [36] | Selective examples: (a) Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77. |
| [36] | (b) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322. |
| [36] | (c) Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527. |
| [37] | Tan, H.; Li, H.; Ji, W.; Wang, L. Angew. Chem. Int. Ed. 2015, 54, 8374. |
| [38] | Conner, E. S.; Crocker, K. E.; Fernando, R. G.; Fronczek, F. R.; Stanley, G. G.; Ragains, J. R. Org. Lett. 2013, 15, 5558. |
| [39] | Shi, Q.; Li, P.; Zhang, Y.; Wang, L. Org. Chem. Front. 2017, 4, 1322. |
| [40] | Wei, W.; Cui, H.; Yang, D.; Yue, H.; He, C.; Zhang, Y.; Wang, H. Green Chem. 2017, 19, 5608. |
| [41] | Yan, J.; Xu, J.; Zhou, Y.; Chen, J.; Song, Q. Org. Chem. Front. 2018, 5, 1483. |
| [42] | Sahoo, H.; Mandal, A.; Dana, S.; Baidya, M. Adv. Synth. Catal. 2018, 360, 1099. |
| [43] | Some examples: (a) Jin, D.-P.; Gao, P.; Chen, D.-Q.; Chen, S.; Wang, J.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2016, 18, 3486. |
| [43] | (b) Wen, J.; Wei, W.; Xue, S.; Yang, D.; Lou, Y.; Gao, C.; Wang, H. J. Org. Chem. 2015, 80, 4966. |
| [44] | Ma, X-L.; Wang, Q.; Feng, X.-Y.; Mo, Z.-Y.; Pan, Y.-M.; Chen, Y.-Y.; Xin, M.; Xu, Y.-L. Green Chem. 2019, 21, 3547. |
| [45] | Some examples: (a) Huang, M.-H.; Hao, W.-J.; Li, G.; Tu, S.-J.; Jiang, B. Chem. Commun. 2018, 54, 10791. |
| [45] | (b) Zhu, S.; Pathigoolla, A.; Lowe, G.; Walsh, D. A.; Cooper, M.; Lewis, W.; Lam, H. W. Chem.-Eur. J. 2017, 23, 17598. |
| [45] | (c) Kawaguchi, S.; Shirai, T.; Ohe, T.; Nomoto, A.; Sonoda, M.; Ogawa, A. J. Org. Chem. 2009, 74, 1751. |
| [45] | (d) Kobiki, Y.; Kawaguchi, S.; Ogawa, A. Tetrahedron Lett. 2013, 54, 545. 9d62fbda-d97a-4485-9efd-c65ff9f5755e |
| [46] | Zhang, Q.; Yuan, P.; Kai, L.; Liu, K.; Ban, Y.; Wang, X.; Wu, L.; Liu, Q. Org. Lett. 2019, 21, 885. |
| [47] | Some examples: (a) Ortgies, S.; Rieger, R.; Rode, K.; Koszinowski, K.; Kind, J.; Thiele, C. M.; Rehbein, J.; Breder, A. ACS Catal. 2017, 7, 7578. |
| [47] | (b) Wilken, M.; Ortgies, S.; Breder, A.; Siewert, I. ACS Catal. 2018, 8, 10901. |
| [47] | (c) Minakata, S.; Morino, Y.; Oderaotoshi, Y.; Komatsu, M. Org. Lett. 2006, 8, 3335. |
| [47] | (d) Nagaraju, K.; Rajesh, N.; Krishna, P. R. Synth. Commun. 2018, 48, 1001. |
| [48] | Some examples: (a) Bin, D.; Wang, H.; Li, J.; Wang, H.; Yin, Z.; Kang, J.; He, B.; Li, Z. Electrochim. Acta 2014, 130, 170. |
| [48] | (b) Yin, Z.; Zheng, Y.; Wang, H.; Li, J.; Zhu, Q.; Wang, Y.; Ma, N.; Hu, G.; He, B.; Knop-Gericke, A.; Schl?gl, R.; Ma, D. ACS Nano. 2017, 11, 12365. |
| [48] | (c) Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230. |
| [48] | (d) Zhang, Y.; Qi, Y.; Yin, Z.; Wang, H.; He, B.; Liang, X.; Li, J.; Li, Z. Green Chem. 2018, 20, 3944. |
| [48] | (e) Sun, K.; Lei, J.; Liu, Y.-J.; Liu, B.; Chen, N. Adv. Synth. Catal. 2020, 362, 1. |
| [49] | Guan, Z.; Wang, Y.; Wang, H.; Huang, Y.; Wang, S.; Tang, H.; Zhang, H.; Lei, A. Green Chem. 2019, 21, 4976. |
| [50] | Some examples: (a) Wang, H.; Li, Y.; Lu, Q.; Yu, M.; Bai, X.; Wang, S.; Cong, H.; Zhang, H.; Lei, A. ACS Catal. 2019, 93, 1888. |
| [50] | (b) Sun, L.; Yuan, Y.; Yao, M.; Wang, H.; Wang, D.; Gao, M.; Chen, Y.-H.; Lei, A. Org. Lett. 2019, 21, 1297. |
| [50] | (c) Zhang, Q.-B.; Yuan, P.-F.; Kai, L.-L.; Liu, K.; Ban, Y.-L.; Wang, X.-Y.; Wu, L.-Z.; Liu, Q. Org. Lett. 2019, 21, 885. |
| [51] | Mallick, S.; Baidya, M.; Mahanty, K.; Maiti, D.; De Sarkar, S. Adv. Synth. Catal. 2020, 362, 1046. |
| [52] | Hua, J.-W.; Fang, Z.; Xu, J.; Bian, M.-X.; Liu, C.-K.; He, W.; Zhu, N.; Guo, K. Green Chem. 2019, 21, 4706. |
| [53] | Some examples: (a) Dakova, B.; Lamberts, L.; Evers, M. Electrochim. Acta 1994, 39, 2363. |
| [53] | (b) Kunai, A.; Harada, J.; Izumi, J.; Tachihara, H.; Sasaki, K. Electrochim. Acta 1983, 28, 1361. |
| [53] | (c) Torii, S.; Uneyama, K.; Ono, M.; Bannou, T. J. Am. Chem. Soc. 1981, 103, 4606. |
| [54] | Hua, J.-W.; Fang, Z.; Bian, M.-X.; Ma, T.; Yang, M.; Xu, J.; Liu, C.-K.; He, W.; Zhu, N.; Yang, Z.; Guo, K. ChemSusChem 2020, 13, 2053. |
| [55] | Some examples: (a) Torii, S.; Uneyama, K.; Ono, M.; Bannou, T. J. Am. Chem. Soc. 1981, 103, 4606. |
| [55] | (b) Dakova, B.; Lamberts, L.; Evers, M. Electrochim. Acta 1994, 39, 2363. |
| [55] | (c) Sun, K.; Wang, X.; Fu, F.; Zhang, C.; Chen, Y.; Liu, L. Green Chem. 2017, 19, 1490. |
| [55] | (d) Kim, Y. J.; Kim, D. Y. Org. Lett. 2019, 21, 1021. |
| [55] | (e) Yang, X.-H.; Ouyang, X.-H.; Wei, W.-T.; Song, R.-J.; Li, J.-H. Adv. Synth. Catal. 2015, 357, 1161. |
| [55] | (f) Reddy, C. R.; Yarlagadda, S.; Ramesh, B.; Reddy, M. R.; Sridhar, B.; Reddy, B. V. S. Eur. J. Org. Chem. 2017, 2017, 2332. |
| [56] | Guo, W.-S.; Wen, L.-R.; Li, M.; Wu, A.-G.; Liu, P.; Pan, C. Chin. J. Org. Chem. 2020, 40, 2855. (in Chinese) |
| [56] | (郭维斯, 文丽荣, 李明, 武安国, 刘鹏, 潘超, 有机化学, 2020, 40, 2855.) |
| [57] | Yue, D.; Larock, R. C. J. Org. Chem. 2002, 67, 1905. |
| [58] | Miao, M.; Huang, X. J. Org. Chem. 2009, 74, 5636. |
| [59] | Gai, R.; Schumacher, R. F.; Back, D. F.; Zeni, G. Org. Lett. 2012, 14, 6072. |
| [60] | Liu, J.; Li, P.; Chen, W.; Wang, L. Chem. Commun. 2012, 48, 10052. |
| [61] | Some examples: (a) Wilhelm, E. A.; Jesse, C. R.; Prigol, M.; Alves, D.; Schumacher, R. F.; Nogueira, C. W. Cell Biol. Toxicol. 2010, 26, 569. |
| [61] | (b) Wilhelm, E. A.; Gai, B. M.; Souza, A. C. G.; Bortolatto, C. F.; Roehrs, J. A.; Nogueira, C. W. Mol. Cell. Biochem. 2012, 365, 175. 5fbc4aab-1fbe-4231-b193-e7cb3c4e5f06 |
| [62] | Pistoia, R. P.; Roehrs, J. A.; Back, D. F.; Zeni, G. Adv. Synth. Catal. 2015, 357, 3655. |
| [63] | Li, X.; He, P.; Zhou, H.; Dong, C. Org. Biomol. Chem. 2018, 16, 2150. |
| [64] | Wang, H.; Ying, J.; Lai, M.; Qi, X.; Peng, J.; Wu, X. Adv. Synth. Catal. 2018, 360, 1693. |
| [65] | Fang, J.; Yan, X.; Zhou, L.; Wang, Y.; Liu, X. Adv. Synth. Catal. 2019, 361, 1985. |
| [66] | Some examples: (a) Sahoo, H.; Mandal, A.; Dana, S.; Baidya, M. Adv. Synth. Catal. 2018, 360, 1099. |
| [66] | (b) Sahoo, H.; Singh, S.; Baidya, M. Org. Lett. 2018, 20, 3678. |
| [67] | Some examples: (a) Bravo, A.; Bj?rsvik, H.-R.; Fontana, F.; Liguori, L.; Minisci, F. J. Org. Chem. 1997, 62, 3849. |
| [67] | (b) Shchepin, R.; M?ller, M. N.; Kim, H.; Hatch, D. M.; Bartesaghi, S.; Kalyanaraman, B.; Radi, R.; Porter, N. A. J. Am. Chem. Soc. 2010, 132, 17490. |
| [67] | (c) Hua, H.-L.; He, Y.-T.; Qiu, Y.-F.; Li, Y.-X.; Song, B.; Gao, P.; Song, X.-R.; Guo, D.-H.; Liu, X.-Y.; Liang, Y.-M. Chem.-Eur. J. 2015, 21, 1468. |
| [67] | (d) Smith, L. M.; Aitken, H. M.; Coote, M. L. Acc. Chem. Res. 2018, 51, 2006. |
| [68] | Selected references: (a) Studer, A. Chem.-Eur. J. 2001, 7, 1159. |
| [68] | (b) Studer, A. Chem. Soc. Rev. 2004, 33, 267. |
| [68] | (c) Li, D.; Li, Y.; Yu, W. Synthesis 2017, 49, 4283. |
| [69] | Sahoo, H.; Grandhi, G. S.; Ramakrishna, I.; Baidya, M. Org. Biomol. Chem. 2019, 17, 10163. |
/
| 〈 |
|
〉 |