

Chinese Journal of Organic Chemistry >
Application of Organocatalysis in Asymmetric Construction of Nitrogen-Containing Heterocyclic Compounds
Received date: 2020-08-20
Revised date: 2020-09-22
Online published: 2020-10-12
Supported by
the National Natural Science Foundation of China(Nos. 21576296); the National Natural Science Foundation of China(21776318); the National Natural Science Foundation of China(81703365)
Nitrogen-containing heterocyclic structures are widely encountered in natural products and pharmaceuticals, which demonstrate broad-spectrum biological and pharmacological activities, and have attracted intensive attention from organic chemists and medicinal chemists. As a consequence, developing their green and efficient pathways, especially in asymmetric fashion, has always been a hot research field in the synthetic chemistry. In recent years, a series of organocatalysts based on amino acid such as proline and pyroglutamic acid have been reported, which have been well applied in the asymmetric construction of various nitrogen-containing compounds. This account mainly focuses on the research of asymmetric synthesis of nitrogen-containing heterocyclic compounds in our group, and the possible mechanisms of some typical reactions are thus discussed.
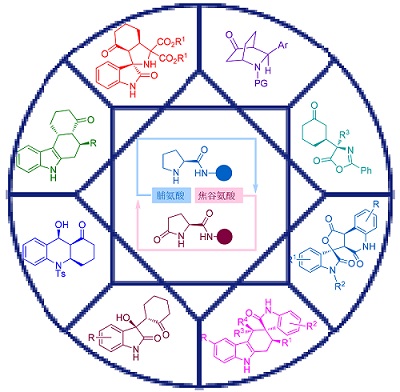
Key words: organocatalysis; asymmetric synthesis; N-heterocycle; proline; pyroglutamic acid
Yu Zheng , Zhenzhen Xie , Kai Chen, , Haoyue Xiang, , Hua Yang, . Application of Organocatalysis in Asymmetric Construction of Nitrogen-Containing Heterocyclic Compounds[J]. Chinese Journal of Organic Chemistry, 2021 , 41(1) : 1 -11 . DOI: 10.6023/cjoc202008037
| [1] | (a) Wang, S.-Q, Huang, W.-Y, Zhang, X.-R, Zhang, X.-T, Pan. C.-X. Chin. J. Org. Chem. 2020, 40, 959. |
| [1] | ( 王淑琴, 黄婉云, 张小蓉, 张晓婷, 潘成学, 有机化学, 2020, 40, 959.). |
| [1] | (b) Li Q.; Wang Y.; Hu M.-J; Chen P.; You W.-W; Zhao P.-L. Chin. J. Org. Chem. 2017, 37, 967. |
| [1] | ( 黎秋, 汪雨, 胡孟金, 陈鹏, 游文玮, 赵培亮, 有机化学, 201 7, 37, 967.). |
| [1] | (c) White N.J.; Pukrittayakamee S.; Phyo A.P.; Rueangweerayut R.; Nosten F.; Jittamala P.; Jeeyapant A.; Jain J.P.; Lefevre G.; Li R.; Magnusson B.; Diagana T.T.; Leong F.J. N. Engl. J. Med. 2014, 371, 403. |
| [1] | (d) Witherup K.M.; Ransom R.W.; Graham A.C.; Bernard A.M.; Salvatore M.J.; Lumma W.C.; Anderson P.S.; Pitzenberger S.M.; Varga S.L. J. Am. Chem. Soc. 1995, 117, 6682. |
| [1] | (e) Güller R.; Borschberg H.-J. Tetrahedron :Asymmetry 1992, 3, 1197. |
| [2] | Vitaku E.; Smith D.T.; Njardarson J.T. J. Med. Chem. 2014, 57, 10257. |
| [3] | Sun G.; Zhang, W.-W; Zhan, X.-P; Liu, Z.-L; Mao. Z.-M.Chin. J. Org. Chem. 2014, 34, 546. |
| [3] | ( 孙广龙, 张蔚蔚, 詹晓平, 刘增路, 毛振民, 有机化学, 2014, 34, 546.). |
| [4] | Smith M.C.; Riskin B.J. Drugs 1991, 42, 365. |
| [5] | (a) Jui N.T.; Garber J.A.; Finelli F.G.; MacMillan D.W. J. Am. Chem. Soc. 2012, 134, 11400. |
| [5] | (b) Kwiatkowski P.; Beeson T.D.; Conrad J.C.; MacMillan D.W. J. Am. Chem. Soc. 2011, 133, 1738. |
| [6] | (a) Schreyer L.; Properzi R.; List B. Angew. Chem. Int. Ed. 2019, 58, 12761. |
| [6] | (b) Ouyang J.; Kennemur J.L.; De C.K.; Fares C.; List B. J. Am. Chem. Soc. 2019, 141, 3414. |
| [7] | (a) Hu B.; Bezpalko M.W.; Fei C.; Dickie D.A.; Foxman B.M.; Deng L. J. Am. Chem. Soc. 2018, 140, 13913. |
| [7] | (b) Tian S.-K.; Chen Y.; Hang J.; Tang L.; McDaid P.; Deng L. Acc. Chem. Res. 2004, 37, 621. |
| [8] | (a) Zhu Y.; Wang Q.; Cornwall R.G.; Shi Y. Chem. Rev. 2014, 114, 8199. |
| [8] | (b) Wong O.A.; Shi Y. Chem. Rev. 2008, 108, 3958. |
| [9] | (a) Li Q.; Levi S.M.; Jacobsen E.N. J. Am. Chem. Soc. 2020, 142, 11865. |
| [9] | (b) Doyle A.G.; Jacobsen E.N. Chem. Rev. 2007, 107, 5713. |
| [10] | (a) Dutartre M.; Bayardon J.; Juge S. Chem. Soc. Rev. 2016, 45, 5771. |
| [10] | (b) James T.; van Gemmeren M.; List B. Chem. Rev. 2015, 115, 9388. |
| [10] | (c) Melchiorre P.; Marigo M.; Carlone A.; Bartoli G. Angew. Chem. Int. Ed. 2008, 47, 6138. |
| [10] | (d) Enders D.; Niemeier O.; Henseler A. Chem. Rev. 2007, 107, 5606. |
| [10] | (e) Kunz R.K.; MacMillan. J. Am. Chem. Soc. 2005, 127, 3240. |
| [11] | (a) Wang L.; Liu J. Synthesis 2016, 49, 960. |
| [11] | (b) Mukherjee S.; Yang J.W.; Hoffmann S.; List B. Chem. Rev. 2007, 107, 5471. |
| [11] | (c) List B.; Lerner R.A.; Barbas C.F. J. Am. Chem. Soc. 2000, 122, 2395. |
| [12] | (a) Reyes-Rodriguez G.J.; Rezayee N.M.; Vidal-Albalat A.; Jorgensen K.A. Chem. Rev. 2019, 119, 4221. |
| [12] | (b) Jensen K.L.; Dickmeiss G.; Jiang H.; Albrecht L.; Jorgensen K.A. Acc. Chem. Res. 2012, 45, 248. |
| [13] | Silvi M.; Verrier C.; Rey Y.P.; Buzzetti L.; Melchiorre P. Nat. Chem. 2017, 9, 868. |
| [14] | Hartikka A.; Arvidsson P.R. Tetrahedron : Asymmetry 2004, 15, 1831. |
| [15] | (a) Enders D.; Grondal C.; Huttl M.R. Angew. Chem. Int. Ed. 2007, 46, 1570. |
| [15] | (b) Cordova A.; Watanabe S.; Tanaka F.; Notz W.; Barbas C.F. J. Am. Chem. Soc. 2002, 124, 1866. |
| [16] | Yang H.; Carter R.G. Org. Lett. 2008, 10, 4649. |
| [17] | Yang H.; Carter R.G. J. Org. Chem. 2009, 74, 2246. |
| [18] | (a) Zaminer J.; Brockmann C.; Huy P.; Opitz R.; Reuter C.; Beyermann M.; Freund C.; Muller M.; Oschkinat H.; Kuhne R.; Schmalz H.G. Angew. Chem. Int. Ed. 2010, 49, 7111. |
| [18] | (b) Bateman L.; Breeden S.W.; O’Leary P. Tetrahedron: Asymmetry 2008, 19, 391. |
| [19] | Chen X.-Y.; Gao Z.-H.; Ye S. Acc. Chem. Res. 2020, 53, 690. |
| [20] | (a) Davies A.T.; Pickett P.M.; Slawin A.M.Z.; Smith A.D. ACS Catal. 2014, 4, 2696. |
| [20] | (b) Massey R.S.; Collett C.J.; Lindsay A.G.; Smith A.D.; O'Donoghue A.C. J. Am. Chem. Soc. 2012, 134, 20421. |
| [21] | (a) Yarlagadda S.; Sankaram G.S.; Balasubramanian S.; Subba Reddy, B.V.Org. Lett. 2018, 20, 4195. |
| [21] | (b) Liu H.; Cun L.-F.; Mi A.-Q.; Jiang Y.-Z.; Gong L.-Z. Org. Lett. 2006, 8, 6023. |
| [22] | (a) Banerjee T.S.; Paul S.; Sinha S.; Das S. Bioorg. Med. Chem. 2014, 22, 6062. |
| [22] | (b) Wishka D.G.; Walker D.P.; Yates K.M.; Reitz S.C.; Jia S.; Myers J.K.; Olson K.L.; Jacobsen E.J.; Wolfe M.L.; Groppi V.E.; Hanchar A.J.; Thornburgh B.A.; Cortes-Burgos L.A.; Wong E.H.; Staton B.A.; Raub T.J.; Higdon N.R.; Wall T.M.; Hurst R.S.; Walters R.R.; Hoffmann W.E.; Hajos M.; Franklin S.; Carey G.; Gold L.H.; Cook K.K.; Sands S.B.; Zhao S.X.; Soglia J.R.; Kalgutkar A.S.; Arneric S.P.; Rogers B.N. J. Med. Chem. 2006, 49, 4425. |
| [23] | Liu H.; Cun L.F.; Mi A.Q.; Jiang Y.Z.; Gong L.Z. Org. Lett. 2006, 8, 6023. |
| [24] | Yang H.; Carter R.G. J. Org. Chem. 2009, 74, 5151. |
| [25] | (a) Nath R.; Pathania S.; Grover G.; Akhtar M.J. J. Mol. Struct. 2020, 1222, 128900. |
| [25] | (b) Arun Y.; Saranraj K.; Balachandran C.; Perumal P.T. Eur. J. Med. Chem. 2014, 74, 50. |
| [26] | (a) Zhang L.-L.; Zhang J.-W.; Xiang S.-H.; Guo Z.; Tan B. Chin. J. Chem. 2018, 36, 1182. |
| [26] | (b) Mei G.-J.; Shi F. Chem. Commun. 201 8, 54, 6607. |
| [26] | (c) Tan F.; Xiao W.-J; Zeng G.-P. Chin. J. Org. Chem. 2017, 37, 824. |
| [26] | ( 谭芬, 肖文精, 曾国平, 有机化学, 201 7, 37, 824.). |
| [26] | (d) Xiao, Y.-L; Zhou, Y.; Wang, J.; Wang, J.-X; Liu, H. Chin. J. Org. Chem. 2015, 35, 2035. |
| [26] | ( 肖永龙, 周宇, 王江, 王进欣, 柳红, 有机化学, 2015, 35, 2035.). |
| [27] | (a) Shi F.; Zhu R.-Y.; Liang X.; Tu S.-J. Adv. Synth. Catal. 2013, 355, 2447. |
| [27] | (b) Chen X.-H.; Wei Q.; Luo S.-W.; Xiao H.; Gong L.-Z. J. Am. Chem. Soc. 2009, 131, 13819. |
| [28] | Shi F.; Tao Z.-L.; Luo S.-W.; Tu S.-J.; Gong L.-Z. Chem. Eur. J. 2012, 18, 6885. |
| [29] | Tian L.; Hu X.-Q.; Li Y.-H.; Xu P.-F. Chem. Commun. 2013, 49, 7213. |
| [30] | Xiao J.-A.; Liu Q.; Ren J.-W.; Liu J.; Carter R.G.; Chen X.-Q.; Yang H. Eur. J. Org. Chem. 2014, 2014, 5700. |
| [31] | Ren J.-W.; Zhou Z.-F.; Xiao J.-A.; Chen X.-Q.; Yang H. Eur. J. Org. Chem. 2016, 2016, 1264. |
| [32] | (a) Wang T.; Yu Z.; Hoon D.L.; Phee C.Y.; Lan Y.; Lu Y. J. Am. Chem. Soc. 2016, 138, 265. |
| [32] | (b) Wei X.; Liu D.; An Q.; Zhang W. Org. Lett. 2015, 17, 5768. |
| [32] | (c) Wang B.; Tu Y.Q. Acc. Chem. Res. 2011, 44, 1207. |
| [33] | (a) Alba A.N.; Rios R. Chem. Asian. J. 2011, 6, 720. |
| [33] | (b) Fisk J.S.; Mosey R.A.; Tepe J.J. Chem. Soc. Rev. 2007, 36, 1432. |
| [34] | Zhang J.; Liu X.; Wu C.; Zhang P.; Chen J.; Wang R. Eur. J. Org. Chem. 2014, 2014, 7104. |
| [35] | Wei Y.; Liu Z.; Wu X.; Fei J.; Gu X.; Yuan X.; Ye J. Chem. Eur. J. 2015, 21, 18921. |
| [36] | Zhang S.-Y.; Ruan G.-Y.; Geng Z.-C.; Li N.-K.; Lv M.; Wang Y.; Wang X.-W. Org. Biomol. Chem. 201 5, 13, 5698. |
| [37] | Wang C.-M.; Xiao J.-A.; Wang J.; Wang S.-S.; Deng Z.-X.; Yang H. J. Org. Chem. 2016, 81, 8001. |
| [38] | (a) Gabriel I. Molecules 2020, 25, 1480. |
| [38] | (b) Gensicka-Kowalewska M.; Cholewiński G.; Dzierzbicka K. RSC Adv. 2017, 7, 15776. |
| [39] | (a) Mayekar N.V.; Nayak S.K.; Chattopadhyay S. Synth. Commun. 2004, 34, 3111. |
| [39] | (b) Jacob R.G.; Perin G.; Botteselle G.V.; Lenardão E.J. Tetrahedron Lett. 2003, 44, 6809. |
| [40] | (a) Chen M.-W.; Cai X.-F.; Chen Z.-P.; Shi L.; Zhou Y.-G. Chem. Commun. 2014, 50, 12526. |
| [40] | (b) Dickmeiss G.; Jensen K.L.; Worgull D.; Franke P.T.; Jorgensen K.A. Angew. Chem. Int. Ed. 2011, 123, 1681. |
| [41] | Li S.; Wang J.; Xia P.-J.; Zhao Q.-L.; Wang C.-M.; Xiao J.-A.; Chen X.-Q.; Xiang H.-Y.; Yang H. J. Org. Chem. 2018, 83, 12284. |
| [42] | Ren J.-W.; Wang J.; Xiao J.-A.; Li J.; Xiang H.-Y.; Chen X.-Q.; Yang H. J. Org. Chem. 2017, 82, 6441. |
| [43] | Ren J.-W.; Zheng L.; Ye Z.-P.; Deng Z.-X.; Xie Z.-Z.; Xiao J.-A.; Zhu F.-W.; Xiang H.-Y.; Chen X.-Q.; Yang H. Org. Lett. 2019, 21, 2166. |
| [44] | Wang J.; Deng Z.-X.; Wang C.-M.; Xia P.-J.; Xiao J.-A.; Xiang H.-Y.; Chen X.-Q.; Yang H. Org. Lett. 2018, 20, 7535. |
/
| 〈 |
|
〉 |