

Chinese Journal of Organic Chemistry >
Research Progress of Polyfluoroalkylation Reaction under Electrochemical Catalysis
Received date: 2020-09-11
Revised date: 2020-09-22
Online published: 2020-10-15
Supported by
Youth Reserve Talent Project of Harbin Business University(2019CX36)
Fluorine chemistry has been widely used in all walks of life, and the combination of fluorine chemistry and organic chemistry is blooming everywhere. Since the introduction of fluorine atoms or fluorine groups into drugs is of great significance, it is essential to seek an effective fluoroalkylation pathway. With the development of electrochemistry, people combine electrochemistry and fluoroalkylation reaction skillfully. In turn, a safer, more economical, environmentally friendly and efficient fluoroalkylation pathway is obtained. The fluoroalkylation reaction pathway under the guidance of electrochemistry has not only reformed the reaction method, but also has advantages in terms of substrate universality. Fluoroalkylation of unsaturated aliphatic compounds their derivatives and aromatic compounds under electrochemical catalysis has been reported. According to the nature of the substrate and its reaction mechanism, the progress of electrocatalytic fluoroalkylation methods is summarized.
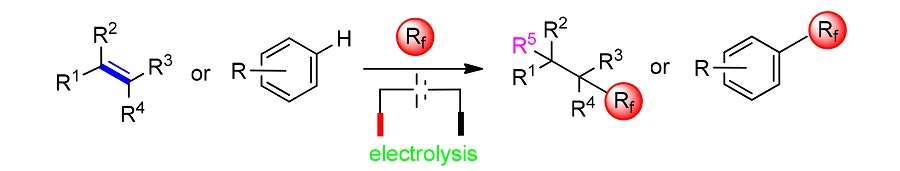
Key words: electrochemistry; fluorochemistry; fluoroalkylation reaction; olefin; aromatic
Yingjie Liu , Yinghui Han , Liqing Lin , Ying Xu . Research Progress of Polyfluoroalkylation Reaction under Electrochemical Catalysis[J]. Chinese Journal of Organic Chemistry, 2021 , 41(3) : 934 -946 . DOI: 10.6023/cjoc202008017
| [1] | He, S. C.; Tian, D. Z.; Guo, B. H.; Chen, H. C. Zhejiang Chem. Ind. 2014, 45, 1. (in Chinese) |
| [1] | (何双材, 田端正, 郭本辉, 陈慧闯, 浙江化工, 2014, 45, 1.) |
| [2] | Kochervinskii, V. V.; Shoranova, L. O.; Shakirzyanov, R. I. Pharm. Sci. Technol. Today 2016,27266. |
| [3] | Tomilenko, A. A.; Bul'bak, T. A.; Pokhilenko, L. N. Dokl. Akad. Nauk 2016, 469, 82. |
| [4] | Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881. |
| [5] | Hagmann, W. K. J. Med. Chem. 2008, 51, 4359. |
| [6] | Kirk, K. L. Org. Process Res. Dev. 2008, 12, 305. |
| [7] | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320. |
| [8] | Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475. |
| [9] | Zhou, Y.; Wang, J.; Gu, Z, N.; Wang, S. N.; Zhu, W. Chem. Rev. 2016, 116, 422. |
| [10] | Wang, J.; Sanchez-Roselló, M.; Acen?, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432. |
| [11] | (a) Li, G. B.; Zhang, C.; Song, C.; Ma, Y. D. Beilstein J. Org. Chem. 2018, 14, 155. |
| [11] | (b) Alonso, C.; Marigorta, E. M.; Rubiale, G.; Palacios, F. Chem. Rev. 2015, 115, 1847. |
| [11] | (c) Studer, A. Angew. Chem.. Int. Ed. 2012, 51, 8950. |
| [12] | (a) Nagib, D. A.; MacMillan, D. W. Nature 2011, 480, 224. |
| [12] | (b) Ji, Y.; Brueckl, T.; Baxter, R.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 14411. |
| [12] | (c) Brien, A. G.; Maruyama, A.; Inokuma, Y.; Fujita, M.; Baran, P. S.; Blackmond, D. G. Angew. Chem.. Int. Ed. 2014, 53, 11868. |
| [13] | (a) Sladojevich, F.; McNeill, E.; Borgel, J.; Zheng, S. L.; Ritter, T. Angew. Chem.. Int. Ed. 2015, 54, 3712. |
| [13] | (b) Cui, L.; Matusaki, Y.; Tada, N.; Miura, T.; Uno, B.; Itoh, A. Adv. Synth. Catal. 2013, 355, 2203. |
| [14] | Cho, E. J.; Senecal, T. D.; Kinzel, T.; Zhang, Y.; Watson, D. A.; Buchwald, S. L. Science 2010, 328, 1679. |
| [15] | (a) Oishi, M.; Kondo, H.; Amii, H. Chem. Commun. 2009,1909. |
| [15] | (b) Shimizu, R.; Egami, H.; Nagi, T.; Chae, J.; Hamashima, Y.; Sodeoka, M. Tetrahedron Lett. 2010, 51, 5947. |
| [15] | (c) Liu, T.; Shen, Q. Org. Lett. 2011, 13, 2342. |
| [15] | (d) Ye, Y.; Sanford, M. S. J. Am. Chem. Soc. 2012, 134, 9034. |
| [16] | (a) Sawada, H.; Nakayama, M.; Yoshida, T. J. Fluorine Chem. 1990, 46, 423. |
| [16] | (b) Langlois, B. R.; Laurent, E.; Roidot, N. Tetrahedron Lett. 1991, 32, 7525. |
| [16] | (c) Kino, T.; Nagase, Y.; Ohtsuka, Y.; Yamamoto, K.; Uraguchi, D.; Tokuhisa, K.; Yamakawa, T. J. Fluorine Chem. 2010, 131, 98. |
| [17] | Wang, X.; Truesdale, L.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 3648. |
| [18] | He, L.; Natte, K.; Rabeah, J.; Taeschler, C.; Neumann, H.; Bruckner, A.; Beller, M. Angew. Chem., Int. Ed. 2015, 54, 4320. |
| [19] | Mohle, S.; Zirbes, M.; Rodrigo, E.; Gieshoff, T.; Wiebe, A.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2018, 57, 6018. |
| [20] | Frontana-Uribe, B. A.; Little, R. D.; Ibanez, J. G.; Palma, A.; Vasquez-Medrano, R. Green Chem. 2010, 12, 2099. |
| [21] | Moeller, K. D. Tetrahedron 2000, 56, 9527. |
| [22] | Sperry, J. B.; Wright, D. L. Chem. Soc. Rev. 2006, 35, 605. |
| [23] | (a) Yoshida, J.-I.; Kataoka, K.; Horcajada, R.; Nagaki, A. Chem. Rev. 2008, 108, 2265. |
| [23] | (b) Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230. |
| [24] | Herzog, G. Chromatographia 2016, 79, 521. |
| [25] | Zhang, Y.; Petersen, J. L.; Milsmann, C. J. Am. Chem. Soc. 2016, 138, 13115. |
| [26] | Yeung, K. T.; To, W. P.; Sun, C.; Cheng, G.; Ma, C.; Tong, G. S. M.; Yang, C.; Che, C. M. Angew. Chem., Int. Ed. 2017, 56, 133. |
| [27] | Gazi, S.; Ng, W. K. H.; Ganguly, R.; Moeljadi, A. M. P.; Hirao, H.; Soo, H. S. Chem. Sci. 2015, 6, 7130. |
| [28] | Uneyama, K. Tetrahedron 1991, 47, 555. |
| [29] | Arai, K.; Watts, K.; Wirth, T. ChemistryOpen 2013, 3, 23. |
| [30] | Jud, W.; Kappe, C. O.; Cantillo, D. Chem.-Eur. J. 2018, 24, 17234. |
| [31] | Gregory, S. S.; Lin, S. ACS Catal. 2018, 8, 5175. |
| [32] | Zhang, L. L.; Zhang, G. T.; Wang, P.; Li, Y. L.; Lei, A. W. Org. Lett. 2018, 20, 7396. |
| [33] | Guan, Z. P.; Wang, H. M.; Huang, Y. G.; Wang, Y. K.; Wang, S. C.; Lei, A. W. Org. Lett. 2019, 21, 4619. |
| [34] | Sun, X.; Ma, H. X.; Mei, T. S.; Fang, P.; Hu, Y. L. Org. Lett. 2019, 21, 3167. |
| [35] | Zou, Z. L.; Zhang, W. G.; Wang, Y.; Kong, L. Y.; Karotsis, G.; Wang, Y. Org. Lett. 2019, 21, 1857. |
| [36] | (a) Zhang, Y. C.; Han, X. L.; Zhao, J. Q.; Qian, Z. J.; Li, T. M.; Tang, Y. Q.; Zhang, H. Y. Adv. Synth. Catal. 2018, 360, 2659. |
| [36] | (b) Wu, Z.; Wang, D. P.; Liu, Y.; Huan, L. T.; Zhu, C. J. Am. Chem. Soc. 2017, 139, 1388. |
| [36] | (c) Ye, K. Y.; Pombar, G.; Fu, N. K.; Sauer, G. S.; Keresztes, I.; Lin, S. J. Am. Chem. Soc. 2018, 140, 2438. |
| [36] | (d) Sauer, G. S.; Lin, S. ACS Catal. 2018, 8, 5175. |
| [36] | (e) Yang, Y. D.; Lwamoto, K.; Tokunaga, E.; Shibata, N. Chem. Commun. 2013, 49, 5510. |
| [36] | (f) Sakamoto, R.; Kashiwagi, H.; Selvakumar, S.; Moteki, S.; Maruoka, K. Org. Biomol. Chem. 2016, 14, 6417. |
| [37] | Wang, H.; Xu, Q.; Yu, S. Y. Org. Chem. Front. 2018, 5, 2224. |
| [38] | Jung, H. I.; Kim, Y.; Kim, Y. D. Org. Biomol. Chem. 2019, 17, 25. |
| [39] | (a) Wu, Q. Y.; Ao, G. Z. Org. Chem. Front. 2018, 5, 2061. |
| [39] | (b) Ye, K. Y.; Pombar, N. F.; Sauer, G. S.; Keresztes, I.; Lin, S. J. Am. Chem. Soc. 2018, 140, 2438. |
| [39] | (c) Jiang, Y. Y.; Dou, G. Y.; Xu, K.; Zeng, C. C. Org. Chem. Front. 2018, 5, 2573. |
| [39] | (d) Ye, K. Y.; Song, Z.; Sauer, S.; Harenberg, H.; Fu, N.; Lin, S. Chem.-Eur. J. 2018, 24, 12274. |
| [39] | (e) Jud, W.; Kappe, C. O.; Cantillo, D. Chem.-Eur. J. 2018, 24, 17234. |
| [39] | (f) Zhang, L.; Zhang, G.; Wang, Y.; Lei, A. Org. Lett. 2018, 20, 7396. |
| [39] | (g) Yingchao, J. W.; Guillot, D. R.; Kouklovsky, C.; Vencent, G. J. Am. Chem. Soc. 2019, 141, 2831. |
| [39] | (h) Zhang, Z.; Zhang, Y.; Cao, F. L.; Bai, G.; Yang, Y.; Mo, F. Org. Lett. 2019, 21, 761. |
| [39] | (i) Ruan, Z.; Huang, Z.; Xu, Z.; Tian, M. X.; Yu, X. Y.; Ackermann, L. Org. Lett. 2019, 21, 1237. |
| [40] | Li, Z.; Jiao, L. C.; Sun, Y. H.; He, Z. Y.; Wei, Z. L.; Liao, W. W. Angew. Chem. 2020, 132, 7333. |
| [41] | (a) Majumdar, K. C.; Mondal, S. Chem. Rev. 2011, 111, 7749. |
| [41] | (b) Iwanrjko, J.; Wojaczynska, E. Org. Biomol. Chem. 2018, 16, 7296. |
| [42] | Dhanak, D.; Duffy, K. J.; Johnston, V. K.; Goerke, J. L.; Darcy, M.; Shaw, A. N.; Gu, B.; Silverman, C.; Gates, A. T.; Nonnemacher, M. R.; Earnshaw, D. L.; Casper, D. J.; Kaura, A.; Baker, A.; Greenwood, C.; Gutshall, L. L.; Maley, D.; Delvecchio, A.; Ricardo, M.; Hofmann, G. A.; Alnoah, Z.; Cheng, H. Y.; Chan, G.; Khandekar, S.; Keenan, R. M.; Sarisky, R. T. J. Biol. Chem. 2002, 277, 38322. |
| [43] | (a) Zheng, J.; Zhang, D. Y.; Cui, S. Adv. Synth. Catal. 2016, 358, 746. |
| [43] | (b) Chen, D.; Ji, M.; Zhu, C. Chem. Commun. 2019, 55, 7796. |
| [44] | Kang, J. C.; Tu, Y. Q.; Dong, J. W.; Chen, C.; Zhou, J.; Ding, T. M.; Zai, J. T.; Chen, Z. M.; Zhang, S. Y. Org. Lett. 2019, 21, 2536. |
| [45] | Zhang, Z. X.; Zhang, L.; Cao, Y.; Li, F.; Bai, G. C.; Liu, G. Q.; Yang, Y.; Mo, F. Y. Org. Let. 2019, 21, 762. |
| [46] | Xiong, P.; Xu, H. H.; Song, J. S.; Xu, H. C. J. Am. Chem. Soc. 2018, 140, 2460. |
| [47] | (a) Studer, A. Angew. Chem., Int. Ed. 2012, 51, 8950. |
| [47] | (b) Ni, C. F.; Hu, M. Y.; Hu, J. B. Chem. Rev. 2015, 115, 765. |
| [47] | (c) Alonso, C.; Marigorta, E. M.; Rubiales, G.; Palacios, F. Chem. Rev. 2015, 115, 1847. |
| [47] | (d) Belhomme, M. C.; Besset, T.; Poisson, T.; Pannecoucke, X. Chem.-Eur. J. 2015, 21, 12836. |
| [47] | (e) Rong, J.; Ni, C. F.; Hu, J. B. J. Org. Chem. 2017, 6, 139. |
| [48] | Taniguchi, T.; Idota, A.; Ishibashi, H. Org. Biomol. Chem. 2011, 9, 3151. |
| [49] | Zhang, S.; Li, L. J.; Zhang, J. J.; Xue, M. Y.; Xu, K. Chem. Sci. 2019, 10, 3181. |
| [50] | Li, F. Y.; lin, D. Z.; He, T. J.; Zhong, W. Q.; Huang, J. M. ChemCatChem 2019, 119, 2350. |
| [51] | Zhao, Y.; Lai, Y. L.; Du, K. S.; Lin, D. Z.; Huang, J. M. J. The J. Org. Chem. 2017, 82, 9655. |
| [52] | (a) Huang, J. M.; Lin, Z. Q.; Chen, D. S. Org. Lett. 2011, 141, 22. |
| [52] | (b) Gong, M.; Huang, J. M. Chem.-Eur. J. 2016, 22, 14293. |
| [52] | (c) Huang, H. B.; Huang, J. M. Adv. Synth. Catal. 2016, 358, 1975. |
| [52] | (d) Lai, Y. L.; Huang, J. M. Org. Lett. 2017, 19, 2022. |
| [52] | (e) Du, K. S.; Huang, J. M. Org. Lett. 2018, 20, 2911. |
| [52] | (f) Lin, D. Z.; Huang, J. M. Org. Lett. 2018, 20, 2112. |
| [52] | (g) Lai, Y. L.; Ye, J. S.; Huang, J. M. Chem.-Eur. J. 2016, 22, 5425. |
| [52] | (h) He, T. J.; Ye, Z.; Ke, Z.; Huang, J. M. Nat. Commun. 2019, 10, 833. |
| [53] | Brien, A. G. O.; Inokuma, M. Y.; Fujita, M.; Baran, P. S.; Blackmond, D. G. Angew. Chem. 2014, 53, 11868. |
| [54] | Qian, P.; Bi, M.; Su, J.; Zha, Z.; Wang, Z. J. Org. Chem. 2016, 81, 4876. |
| [55] | Dudkina, Y. B.; Khrizanforov, M. N.; Gryazonova, T. V.; Budnikova, Y. H. J. Organomet. Chem. 2014, 751, 301. |
| [56] | Dubinina, G. G.; Brennessel, W. W.; Miller, J. L.; Vicic, D. A. Organometallics 2008, 27, 3933. |
| [57] | Hossain, M. J.; Ono, T.; Wakiya, K.; Hisaeda, Y. J. Chem. Commun. 2017, 53, 10878. |
| [58] | Cui, L. X.; Ono, T.; Morita, Y.; Hisaede, Y. Dalton Trans. 2020, 22, 7546. |
| [59] | Jud, W.; Maljuric, S.; Kappe, C. O.; Cantillo, D. Org. Lett. 2019, 21, 7970. |
| [60] | Rodrigo, S.; Um, C.; Mixdorf, J. C.; Gunasekera, D.; Nguyen, H. M.; Luo, L. Org. Lett. 2020, 22, 6719. |
| [61] | Brien, A. G. O.; Inokuma, M.Y. Fujita, M.; Baran, P. S.; Blackmond, D. G. Angew. Chem. 2014, 53, 11868. |
| [62] | Beryl, J. R.; Raj, X. J. Orient. J. Chem. 2018, 34, 2098. |
| [63] | Dou, G. Y.; Jiang, Y. Y.; Xu, K.; Zeng, C. C. Org. Chem. Front. 2019, 6, 2392. |
| [64] | Jiang, Y. Y.; Dou, G. Y.; Xu, K.; Zeng, C. C. Org. Chem. Front. 2018, 5, 2573. |
/
| 〈 |
|
〉 |