

Chinese Journal of Organic Chemistry >
Synthesis and Fungicidal Activity of Novel Allyl Benzoate Compounds Containing Triazole
Received date: 2020-06-19
Revised date: 2020-07-20
Online published: 2020-10-22
Supported by
the Natural Science Foundation of Zhejiang Province(LY19C140002); the Research Fund of Department of Education of Zhejiang Province(Y201941832)
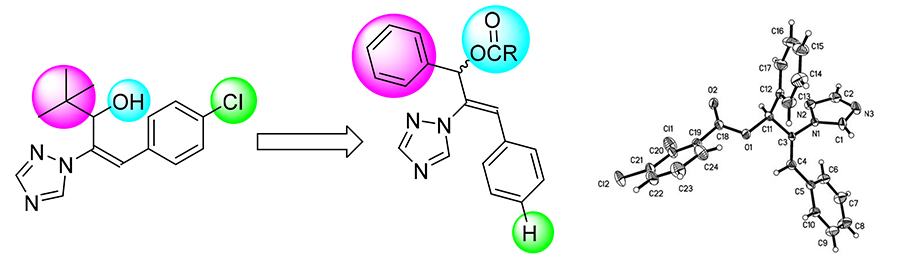
In order to find pesticide lead compounds with high fungicidal activity, a series of unreported allyl benzoate compounds containing triazole were designed and synthesized. The structures were confirmed by 1H NMR and HRMS, and the crystal structure of (R,S)-(Z)-1,3-diphenyl-2-(1H-1,2,4-triazol-1-yl)allyl 2,3-dichlorobenzoate (5j) was determined by X-ray single crystal diffraction. The bioassay results showed that (R,S)-(Z)-1,3-diphenyl-2-(1H-1,2,4-triazol-1-yl)allyl benzoate (5a), (R,S)-(Z)-1,3-diphenyl-2-(1H-1,2,4-triazol-1-yl)allyl 2-fluorobenzoate (5g) and (R,S)-(Z)-1,3-diphenyl-2-(1H-1,2,4-triazol-1- yl)allyl 3-methylbenzoate (5n) exhibited good control efficacy against Sclerotinia sclerotiorummore than 70% at 50 mg/L.
Wei Yu , Han Wang , Li-Jing Min , Xue-Wen Hua , Xing-Hai Liu . Synthesis and Fungicidal Activity of Novel Allyl Benzoate Compounds Containing Triazole[J]. Chinese Journal of Organic Chemistry, 2021 , 41(2) : 826 -832 . DOI: 10.6023/cjoc202006037
| [1] | Hua X.W.; Liu W.R.; Su Y.Y.; Liu X.H.; Liu J.B.; Liu N.N.; Wang G.Q.; Jiao X.Q.; Fan X.Y.; Xue C.M.; Liu Y.; Liu M. Pest Manage. Sci. 2020, 76, 2368. |
| [2] | Cheng L.; Zhang R.R.; Wu H.K.; Liu X.H.; Xu T.M. Front. Chem. Sci. Eng. 2019, 13, 369. |
| [3] | Fu Q.; Cai P.P.; Cheng L.; Zhong L.K.; Tan C.X.; Shen Z.H.; Han L.; Xu T.M.; Liu X.H. Pest Manage. Sci. 2020, 76, 868. |
| [4] | Liu X.H.; Qiao L.; Zhai Z.W.; Cai P.P.; Cantrell C.L.; Tan C.X.; Weng J.Q.; Han L.; Wu H.K. Pest Manage. Sci. 2019, 75, 2892. |
| [5] | Shi Y.J.; Zhou Q.; Wang Y.; Qian H.W.; Ye L.Y.; Feng X.; Chen H.; Li Y.T.; Dai H.; Wei Z.H.; Wu J.M. Chin. J. Org. Chem. 2018, 38, 2450. (in Chinese) |
| [5] | 石玉军, 周钱, 王杨, 钱宏炜, 叶林玉, 冯霞, 陈辉, 李雅婷, 戴红, 魏中昊, 吴锦明, 有机化学, 2018, 38, 2450.). |
| [6] | Liu X.H.; Xu X.Y.; Tan C.X.; Weng J.Q.; Xin J.H.; Chen J. Pest Manage. Sci. 2015, 71, 292. |
| [7] | Liu X.H.; Sun Z.H.; Yang M.Y.; Tan C.X.; Weng J.Q.; Zhang Y.G.; Ma Y. Chem. Biol. Drug Des. 2014, 84, 342. |
| [8] | Guan L.P.; Sui X.; Deng X.Q.; Quan Y.C.; Quan Z.S. Eur. J. Med. Chem. 2010, 45, 1746. |
| [9] | Wang Y.R.; Zheng D.D.; Wang Y.; Ye H.; Yao W.; Ding Y.; Gu H.X.; Feng X.; Li L.; Dai H. Chin. J. Org. Chem. 2019, 39, 2053. (in Chinese) |
| [9] | 王誉蓉, 郑丹丹, 王杨, 叶浩, 姚炜, 丁颖, 顾海鹰, 冯霞, 李玲, 戴红, 有机化学, 2019, 39, 2053.). |
| [10] | Qin X.; Yu H.B.; Dai H.; Qin Z.F.; Zhang X.; Bing G.F.; Wang T.T.; Fang J.X. Chin. Chem. Lett. 2010, 21, 283. |
| [11] | Stoelzer C.; Kraemer W.; Buechel K.H.; Meiser W. DE 2406665, 1975. |
| [12] | Funaki Y.; Oshita H.; Yamamoto S.; Tanaka S.; Kato T. JP 55124771, 1980. |
| [13] | Fletcher R.A.; Hofstra G.; Gao J.G. Plant Cell Physiol. 1986, 27, 367. |
| [14] | Dietz J.; Riggs R.; Boudet N.; Lohmann J.K.; Craig I.R.; Haden E.; Lauterwasser E. M. W.; Mueller B.; Grammenos W.; Grote T. WO 2013007767, 2013. |
| [15] | Ye T.; Ma Z.Q.; Bi Q.Y.; Niu F.S.; Han X.Y.; Zhang X.F.; Wang W.Q.; Zhang L.H. Chin. J. Pestic. Sci. 2012, 14, 1. (in Chinese) |
| [15] | 叶滔, 马志强, 毕秋艳, 牛芳胜, 韩秀英, 张小风, 王文桥, 张利辉, 农药学学报, 2012, 14, 1.). |
| [16] | Gobeil-Richard M.; Tremblay D.M.; Beaulieu C.; Van der Heyden H.; Carisse O. Pest Manage. Sci. 2016, 72, 566. |
| [17] | Birch C. P. D.; Shaw M.W. J. App. Ecol. 1997, 34, 1032. |
| [18] | Liu X.H.; Yu W.; Min L.J.; Wedge D.E.; Tan C.X.; Weng J.Q.; Wu H.K.; Cantrell C.L.; Bajsa-Hischel J.; Hua X.W.; Duke S.O. J. Agric. Food Chem. 2020, 68, 7324. |
| [19] | Hu A.X.; Chen P.; Yang Z.; Wu X.Y.; Chen K. Chin. J. Med. Chem. 2002, 12, 340. (in Chinese) |
| [19] | 胡艾希, 陈平, 杨郑, 伍小云, 陈科, 中国药物化学杂志, 2002, 12, 340.). |
| [20] | Xu L.Z.; Li W.H.; Yu G.P.; Qin Y.Q.; Yang S.H.; Hou B.R. Chem. Res. Chin. Univ. 2005, 21, 528. |
| [21] | Chen S.Q.; Liu F.M. J. Chem. Crystallogr. 2011, 41, 485. |
/
| 〈 |
|
〉 |