

Chinese Journal of Organic Chemistry >
Synthesis and Antitumor Activity of Novel Pyrimidine Monocyclic Nonclassical Antifolates
Received date: 2020-07-02
Revised date: 2020-09-17
Online published: 2020-10-22
Supported by
the National Natural Science Foundation of China(21172014)
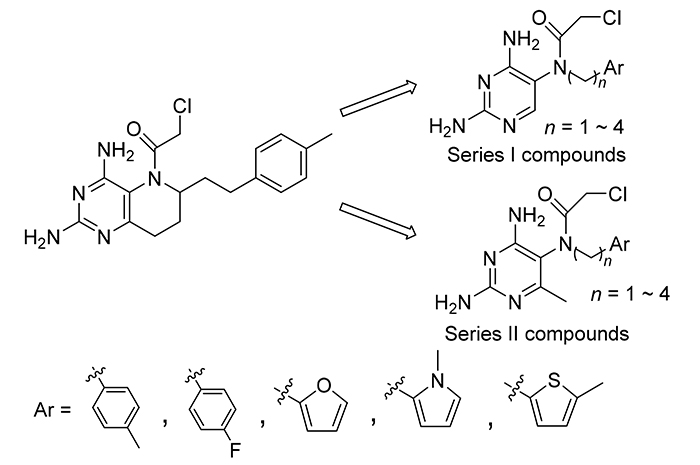
Regarding the nonclassical antifolates 6-(4'-methylphenethyl)-5-chloroacetyl-5,6,7,8-tetrahydropyrido[3,2-d]pyri- midine (wm-8.2) as the lead compound, in order to improve molecular flexibility and simplify molecular structure, two series of compounds were designed and synthesized according to the 6-H and 6-methyl group. And the effects of carbon chain length and aromatic heterocyclic side chain on antitumor activity were investgated. Besides, the activities of key intermediates with molecular skeleton of folic acid inhibitor were measured to study the effect of the chloroacetyl group at N(5) position. Structures of 36 target compounds and key intermediates were confirmed by 1H NMR, 13C NMR and MS. The biological activity results showed that 6-methyl-2,4-diamino-5-(N-(4-methylphenyl)propyl-N-(2-chloroacetyl))aminopyrimidine (6b-3), which had three-carbon bridge and p-methylbenzene ring side chain, exhibited the best inhibition activities against HL-60, A549 and HCT116 cells with IC50 values as 0.25, 0.83 and 0.63 μmol?L –1 respectively. 6-Methyl-2,4-diamino-5-(N-(4-methylphenyl)- propyl)aminopyrimidine (5b-3), the key intermediate of 6b-3, showed excellent dihydrofolate reductase inhibitory activity. Molecular docking studies further explored the structure-activity relationship and possible causes of the difference inhibitory activity against dihydrofolate reductase.
Jing Cong , Fang Fang , Liangmin Xue , Meng Wang , Chao Tian , Xiaowei Wang , Junyi Liu , Zhili Zhang . Synthesis and Antitumor Activity of Novel Pyrimidine Monocyclic Nonclassical Antifolates[J]. Chinese Journal of Organic Chemistry, 2021 , 41(2) : 776 -787 . DOI: 10.6023/cjoc202007006
| [1] | Raz S.; Stark M.; Assaraf Y.G. Drug Resist. Updates 2016, 28, 43. |
| [2] | Lilah R.; Ilan I.; Yotam K.; David G.P.; Gerrit J.; Yehuda G.A. Biochem. J. 2002, 367, 741. |
| [3] | Al-Omary F. A. M.; Hassan G.S.; El-Messery S.M.; Nagi M.N.; El-Subbagh H.I. Eur. J. Med. Chem. 2017, 33, 335. |
| [4] | Wang M.; Tian C.; Xue L.M.; Li H.; Cong J.; Fang F.; Yang J.J.; Yuan M.M.; Chen Y.; Guo Y.; Wang X.W.; Liu J.Y.; Zhang Z.L. Eur. J. Med. Chem. 2020, 190, 112113. |
| [5] | Li H.; Fang F.; Liu Y.Q.; Xue L.M.; Wang M.; Guo Y.; Wang X.W.; Tian C.; Liu J.Y.; Zhan g, Z. L. Bioorg. Med. Chem. 2018, 26, 2674. |
| [6] | Ellis P.A.; Norman A.; Hill A.; O'BrienM M. E. R.; Nicolson M.; Hickish T.; Cunningham D. Eur. J. Cancer 1995, 31, 1594. |
| [7] | Grant S. Adv. Cancer Res. 1997, 72, 197. |
| [8] | Wang M.; Yang J.J.; Yuan M.M.; Xue L.M.; Li H.; Tian C.; Wang X.W.; Liu J.Y.; Zhang Z.L. Eur. J. Med. Chem. 2017, 128, 88. |
| [9] | Fang F.; Xue L.M.; Cong J.; Tian C.; Wang X.W.; Liu J.Y.; Zhang Z.L. Chem. J. Chin. Univ. 2019, 40, 844. (in Chinese) |
| [9] | 方芳, 薛良敏, 丛婧, 田超, 王孝伟, 刘俊义, 张志丽, 高学校化学学报, 2019, 40, 844.). |
| [10] | Abdullah Z; Bakar M.A. J. Chem. Sci. 2006, 4, 241. |
| [11] | Weix D.J.; Dreher S.D.; Katz T.J. J. Am. Chem. Soc. 2000, 122, 10027. |
| [12] | Houjeiry T.I.; Poe S.L.; Mcquade D.T. Org. Lett. 2012, 14, 4394. |
| [13] | Gao T.F.; Zhang C.Y.; Shi X.W.; Guo R.; Zhang K.; Gu J.M.; Li L.; Li S.L.; Zheng Q.Q.; Cui M.Y.; Cui M.; Gao X.M.; Liu Y.; Wang L. Eur. J. Med. Chem. 2019, 178, 329. |
| [14] | El-Subbagh H.I.; Hassan G.S.; El-Messery S.M.; Al-Rashood S.T.; Al-Omary F. A. M.; Abulfadl Y.S.; Shabayek M.I. Eur. J. Med. Chem. 2014, 74, 234. |
| [15] | Ng H.L.; Chen S.Y.; Chew E.H.; Chui W.K. Eur. J. Med. Chem. 2016, 115, 63. |
| [16] | Zhang Z.L.; Tian C.; Zhou S.X.; Wang W.; Guo Y.; Xia J.; Liu Z.M.; Wang B.; Wang X.W.; Golding B.T.; Griff R.J.; Du Y.S.; Liu J.Y. Eur. J. Med. Chem. 2012, 58, 228. |
| [17] | Sadanandam P.; Jyothi V.; Chari M.A.; Das P. Tetrahedron Lett. 2011, 52, 5521. |
| [18] | Landor P.D.; Rydon H.N. J. Chem. Soc. 1955, 11, 1113. |
| [19] | Taylor E.; Barton J. J. Org. Chem. 1959, 24, 127. |
| [20] | Oki M.; Lwamura H.; Onoda T.; Lwamura M. Bull. Chem. Soc. Jpn. 1959, 32, 1135. |
| [21] | An J.; Work D.N.; Kenyon C.; Procter D.J. J. Org. Chem. 2014, 79, 6743. |
| [22] | Jackman L.M.; Haddon V.R. J. Am. Chem. Soc. 1974, 96, 5130. |
| [23] | Solabannavar S.B.; Desai U.V.; Mane R.B. Indian J. Chem., Sect. B : Org. Chem. Incl. Med. Chem. 2004, 43, 2235. |
| [24] | Larionov E.; Lin L.; Gue?ne?e L.; Mazet C. J. Am. Chem. Soc. 2014, 136, 16882. |
| [25] | Bert L. Compt. Rend. Acad. Sci. Paris. 1928, 186, 699. |
| [26] | Kampmeier J.A.; Harris S.H.; Wedegaertner D.K. J. Org. Chem. 1980, 45, 315. |
| [27] | Bhatt V.; Samant S.D.; Pednekar, S. Synth. Commun. 2017, 47, 968. |
| [28] | Keith B.; Andrew C.B. J. Chem. Res., Miniprint 1992, 2, 514. |
| [29] | Gangjee A.; Jain H.D.; Queener S.F.; Kisliuk R.L. J. Med. Chem. 2008, 51, 4589. |
/
| 〈 |
|
〉 |