

Chinese Journal of Organic Chemistry >
Efficient Copper-Catalyzed Domino Synthesis of Phosphonated Isoquinolin-1(2H)-ones Using Cyanomethylphosphonates as Building Blocks
Received date: 2020-08-25
Revised date: 2020-10-18
Online published: 2020-10-22
Supported by
the Natural Science Foundation of Shandong Province(ZR2016JL012); the Scientific Research Foundation of Qingdao University of Science and Technology.()
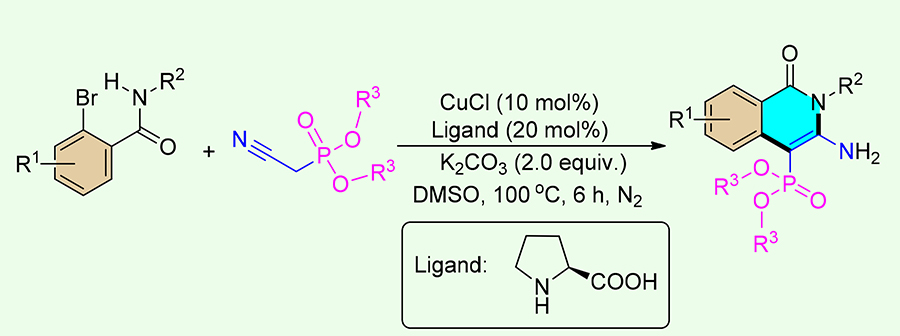
An efficient and convenient copper-catalyzed cascade synthesis of C-4 phosphonated isoquinolin-1(2 H)-ones has been initially proposed. This is the first example for the construction of phosphine-containing heterocycles through copper-catalyzed Ullmann-type coupling reactions using cyanomethylphosphonates as the building blocks, and it will broaden the strategies of organophosphorus synthesis in the field of organic and pharmaceutical chemistry.
Suyan Zhao , Xueqin Gong , Ziyu Gan , Qiuli Yan , Xueliang Liu , Daoshan Yang . Efficient Copper-Catalyzed Domino Synthesis of Phosphonated Isoquinolin-1(2H)-ones Using Cyanomethylphosphonates as Building Blocks[J]. Chinese Journal of Organic Chemistry, 2021 , 41(1) : 258 -266 . DOI: 10.6023/cjoc202008045
| [1] | (a) Ratnayake R.; Lacey E.; Tennant S.; Gill J.H.; Capon R.J. Chem. -Eur. J. 2007, 13, 1610. |
| [1] | (b) Billamboz M.; Bailly F.; Lion C.; Touati N.; Vezin H.; Calmels C.; Andreóla M.-L.; Christ F.; Debyser Z.; Cotelle P. J. Med. Chem. 2011, 54, 1812. |
| [1] | (c) Dickinson R.P.; Bell A.W.; Hitchcock C.A.; Narayana-Swami S.; Ray S.J.; Richardson K.; Troke P.F. Bioorg. Med. Chem. Lett. 1996, 6, 2031. |
| [1] | (d) Jatav V.; Mishra P.; Kashaw S.; Stables J.P. Eur. J. Med. Chem. 2008, 43, 135. |
| [1] | (e) Glushkov V.A.; Shklyaev Y.V. Chem. Heterocycl. Compd. 2001, 37, 66. |
| [2] | (a) Kashaw S.K.; Gupta V.; Kashaw V.; Mishra P.; Stables J.P.; Jain N.K. Med. Chem. Res. 2010, 19, 250. |
| [2] | (b) Wang Z.; He W.-M. Chin. J. Org. Chem. 2019, 39, 3594. (in Chinese) |
| [2] | ( 王峥, 何卫民, 有机化学, 2019, 39, 3594.). |
| [2] | (c) Li X.-L.; Wang J.-Q.; Li L.; Yin Y.-W.; Ye L.-W. Acta Chim. Sinica 2016, 74, 49. (in Chinese) |
| [2] | ( 李新玲, 王佳琪, 李龙, 尹应武, 叶龙武, 化学学报, 2016, 74, 49.). |
| [3] | (a) Horsman G.P.; Zechel D.L. Chem. Rev. 2017, 117, 5704. |
| [3] | (b) Gahungu M.; Arguelles-Arias A.; Fickers P.; Zervosen A.; Joris B.; Damblon C.; Luxen A. Bioorg. Med. Chem. 2013, 21, 4958. |
| [3] | (c) Tang W.; Zhang X. Chem. Rev. 2003, 103, 3029. |
| [3] | (d) Bhattacharya A.K.; Thyagarajan G. Chem. Rev. 1981, 81, 415. |
| [3] | (e) Kirumakki S.; Huang J.; Subbiah A.; Yao J.; Rowland A.; Smith B.; Mukherjee A.; Samarajeewa S.; Clearfield A. J. Mater. Chem. 2009, 19, 2593. |
| [3] | (f) Krylov S.; Kashemirov B.A.; Hilfinger J.M.; McKenna C.E. Mol. Pharmaceutics 2013, 10, 445. |
| [3] | (g) Qiao B.; Cao H.-Q.; Huang Y.-J.; Zhang Y.; Nie J.; Zhang F.-G.; Ma J.-A. Chin. J. Chem. 2018, 36, 809. |
| [4] | (a) Schlummer B.; Scholz U. Adv. Synth. Catal. 2004, 346, 1599. |
| [4] | (b) Hayashi T. Acc. Chem. Res. 2000, 33, 354. |
| [5] | (a) Zhuang R.; Xu J.; Cai Z.; Tang G.; Fang M.; Zhao Y. Org. Lett. 2011, 13, 2110. |
| [5] | (b) Lee H.; Yun J. Org. Lett. 2018, 20, 7961. |
| [5] | (c) Mehellou Y.; Rattan H.S.; Balzarini J. J. Med. Chem. 2018, 61, 2211. |
| [6] | Demmer C.S.; Krogsgaard-Larsen N.; Bunch L. Chem. Rev. 2011, 111, 7981. |
| [7] | Li B.; Yang J.; Xu H.; Song H.; Wang B. J. Org. Chem. 2015, 80, 12397. |
| [8] | (a) Shi W.; Li J.; Su D.; Wang X.; Zhang L.; Pan L.; Wu X.; Wu H. Angew. Chem., Int. Ed. 2019, 58, 1106. |
| [8] | (b) White J.D.; Laura Q.; Wang G. J. Org. Chem. 2007, 72, 1717. |
| [9] | Zhu W.; Zhang D.; Yang N.; Liu H. Chem. Commun. 2014, 50, 10634. |
| [10] | (a) Yang D.; An B.; Wei W.; Tian L.; Huang B.; Wang H. ACS Comb. Sci. 2015, 17, 113. |
| [10] | (b) Yan K.; Yang D.; Wei W.; Lu S.; Li G.; Zhao C.; Zhang Q.; Wang H. Org. Chem. Front. 2016, 3, 66. |
| [10] | (c) Gan Z.; Yan Q.; Li G.; Li Q.; Dou X.; Li G.-Y.; Yang D. Adv. Synth. Catal. 2019, 361, 4558. |
| [11] | (a) Bhunia S.; Pawar G.G.; Kumar S.V.; Jiang Y.; Ma D. Angew. Chem., Int. Ed. 2017, 56, 16136. |
| [11] | (b) Xu S.; Lu J.; Fu H. Chem. Commun. 2011, 47, 5596. |
| [11] | (c) Liu X.; Fu H.; Jiang Y.; Zhao Y. Angew. Chem., Int. Ed. 2009, 48, 348. |
| [11] | (d) Xu L.; Jiang Y.; Ma D. Org. Lett. 2012, 14, 1150. |
| [11] | (e) Ma D.; Cai Q. Acc. Chem. Res. 2008, 41, 1450. |
| [11] | (f) Lv X.; Bao W.L. J. Org. Chem. 2009, 74, 5618. |
| [11] | (g) Martìn R.; Rivero R.; Buchwald S.L. Angew. Chem., Int. Ed. 2006, 45, 7079. |
| [11] | (h) Liu Y.; Wan J.-P. Org. Biomol. Chem. 2011, 9, 6873. |
| [11] | (i) Verma A.K.; Kesharwani T.; Singh J.; Tandon V.; Larock R.C. Angew. Chem., Int. Ed. 2009, 48, 1138. |
| [11] | (j) Moessner C.; Bolm C. Org. Lett. 2005, 7, 2667. |
| [11] | (k) Cheng L.; Ge X.; Liu X.; Feng Y. Chin. J. Org. Chem. 2020, 40, 2008. (in Chinese) |
| [11] | ( 成琳, 葛新, 刘学民, 冯云辉, 有机化学, 2020, 40, 2008.). |
| [11] | (l) Xie J.; Wang X.; Wu F.; Zhang J. Chin. J. Org. Chem. 2019, 39, 3026. (in Chinese) |
| [11] | ( 谢建伟, 汪小创, 吴丰田, 张洁, 有机化学, 2019, 39, 3026.). |
| [12] | (a) Lu B.; Ma D. Org. Lett. 2006, 8, 6115. |
| [12] | (b) Xie X.; Cai G.; Ma D. Org. Lett. 2005, 7, 4693. |
| [12] | (c) Liu T.; Wang R.; Yang H.; Fu H. Chem. -Eur. J. 2011, 17, 6765. |
| [12] | (d) Li M.; Ning J.; Yu L.; Wen L. Chin. J. Org. Chem. 2016, 36, 2715. (in Chinese) |
| [12] | ( 李明, 宁加彬, 于乐, 文丽荣, 有机化学, 2016, 36, 2715.). |
| [12] | (e) Zhao S.; Wang Z.-L. Chin. J. Org. Chem. 2016, 36, 862. (in Chinese) |
| [12] | ( 赵苏艳, 王祖利, 有机化学, 2016, 36, 862.). |
/
| 〈 |
|
〉 |