

Chinese Journal of Organic Chemistry >
Cu(OAc)2-Mediated C—H Bond Dithiolation of Amide-Oxazolines with Aryl Thiols
Received date: 2020-09-12
Revised date: 2020-09-30
Online published: 2020-10-22
Supported by
Key Science Research of Education Committee in Henan Province(19A150035); Key Scienti?c and Technological Project of Henan Province(192102110222); Program for Science & Technology Innovation Talents in Universities of Henan Province(14HASTIT016); Program of Science and Technology Innovation Talents of Henan Province(184100510011)
An efficient copper-mediated dithiolation of C(sp2)—H bonds with aryl thiols was achieved by using amide-oxazo- line as directing group. This strategy gives a variety of functionalized thioethers in moderate to excellent yields (up to 90%) in simple and efficient way. Importantly, the substrate scope is not limited to various substituted phenylamides, and diverse pyridine amides are also compatible. Furthermore, the protocol has been successfully implemented for the gram-scale synthesis as well.
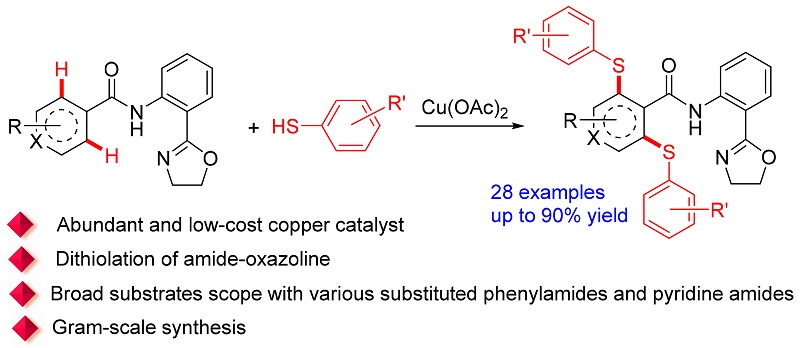
Key words: copper; C—H bond; thiolation; amide-oxazoline; aryl thiols
Tao Wang , Xiaosha Wang , Yawen Song , Jingjing Huo , Jingshuan Zhou , Qingwei Kang , Lantao Liu . Cu(OAc)2-Mediated C—H Bond Dithiolation of Amide-Oxazolines with Aryl Thiols[J]. Chinese Journal of Organic Chemistry, 2021 , 41(3) : 1098 -1107 . DOI: 10.6023/cjoc202009030
| [1] | Fraústo da Silva, J.R.; Williams, R. J. P. The Biological Chemistry of the Elements, Oxford University Press, New York, 2001. |
| [2] | (a) Scott, K. A.; Njardarson, J. T. Top. Curr. Chem. 2018, 376, 5. |
| [2] | (b) Zhao, J.; Jiang, X. Chin. Chem. Lett. 2018, 29, 1079. |
| [3] | Mellah, M.; Voituriez, A.; Schulz, E. Chem. Rev. 2007, 107, 5133. |
| [4] | Cinar, M. E.; Ozturk, T. Chem. Rev. 2015, 115, 3036. |
| [5] | (a) Khanal, H. D.; Kim, S.H. Lee, Y. R. RSC Adv. 2016, 6, 58501. |
| [5] | (b) Dong, Y.-T.; Jin, Q.; Zhou, L.; Chen, J. Org. Lett. 2016, 18, 5708. |
| [5] | (c) Li, Y.; Zhu, F.; Wang, Z.; Wu, X.-F. Chem. Asian J. 2016, 11, 3503. |
| [5] | (d) Yang, Z.-H.; An, Y.-L.; Chen, Y.; Shao, Z.-Y.; Zhao, S.-Y. Adv. Synth. Catal. 2016, 358, 3869. |
| [5] | (e) Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T. S. A.; Liu, X. Chem. Soc. Rev. 2015, 44, 291. |
| [6] | For selected reviews on transition metals catalyzed C—H functionalization, see: (a) Liu, Y.-H.; Xia, Y.-N.; Shi, B.-F. Chin. J. Chem. 2020, 38, 635. |
| [6] | (b) Shang, M.; Sun, S.-Z.; Wang, H.-L.; Wang, M.-M.; Dai, H.-X. Synthesis 2016, 48, 4381. |
| [6] | (c) Rao, W.-H.; Shi, B.-F. Org. Chem. Front. 2016, 3, 1028. |
| [6] | (d) Liu, J.; Chen, G.; Tan, Z. Adv. Synth. Catal. 2016, 358, 1174. |
| [6] | (e) Hu, Y.; Wang, C. Acta Phys.-Chim. Sin. 2019, 35, 913. |
| [7] | (a) Jiang, Y.-J.; Liang, G.-H.; Zhang, C.; Loh, T.-P. Eur. J. Org. Chem. 2016, 2016, 3326. |
| [7] | (b) Iwasaki, M.; Nishihara, Y. Dalton Trans 2016, 45, 15278. |
| [7] | (c) Iwasaki, M.; Kaneshika, W.; Tsuchiya, Y.; Nakajima, K.; Nishihara, Y. J. Org. Chem. 2014, 79, 11330. |
| [7] | (d) Iwasaki, M.; Lyanaga, M.; Tsuchiya, Y.; Nishimura, Y.; Li, W.-J.; Li, Z.-P.; Nishihara, Y. Chem.-Eur. J. 2014, 20, 2459. |
| [8] | (a) Xie, W.-C.; Li, B.; Wang, B.-Q. J. Org. Chem. 2016, 81, 396. |
| [8] | (b) Yang, Y.-X.; Hou, W.; Qin, L.-H.; Du, J.-J.; Feng, H.-J.; Zhou, B.; Li, Y.-C. Chem.-Eur. J. 2014, 20, 416. |
| [9] | (a) Ma, W.; Weng, Z.; Fang, X.; Gu, L.; Song, Y.; Ackermann, L. Eur. J. Org. Chem. 2019, 2019, 41. |
| [9] | (b) Ma, W.; Weng, Z.; Rogge, T.; Gu, Li.; Lin, J.; Peng, A.; Luo, X.; Gou, X.; Ackermann, L. Adv. Synth. Catal. 2018, 360, 704. |
| [9] | (c) Mandal, A.; Dana, S.; Sahoo, H.; Grandhi, G. S.; Baidya, M. Org. Lett. 2017, 19, 2430. |
| [9] | (d) Ma, W.; Dong, H.; Wang, D.; Ackermann, L. Adv. Synth. Catal. 2017, 359, 966. |
| [10] | (a) Gao, F.; Zhu, W.; Zhang, D.-Y.; Li, S.-J.; Wang, J.; Liu, H. J. Org. Chem. 2016, 81, 9122. |
| [10] | (b) Yan, S.-Y.; Liu, Y.-J.; Liu, B.; Liu, Y.-H.; Shi, B.-F. Chem. Commun. 2015, 51, 4069. |
| [10] | (c) Yan, S.-Y.; Liu, Y.-J.; Liu, B.; Liu, Y.-H.; Zhang, Z.-Z.; Shi, B.-F. Chem. Commun. 2015, 51, 7341. |
| [10] | (d) Lin, C.; Yu, W.-L.; Yao, J.-Z.; Wang, B.-J.; Liu, Z.-X.; Zhang, Y.-H. Org. Lett. 2015, 17, 1340. |
| [10] | (e) Wang, X.; Qiu, R.-H.; Yan, C.-Y.; Reddy, V. P.; Zhu, L.-Z.; Xu, X.-H.; Yin, S.-F. Org. Lett. 2015, 17, 1970. |
| [11] | Chen, X.; Hao, X.-S.; Goodhue, C. E.; Yu, J.-Q. J. Am. Chem. Soc. 2006, 128, 6790. |
| [12] | Chu, L.-L.; Yue, X.-Y.; Qing, F.-L. Org. Lett. 2010, 12, 1644. |
| [13] | Tran, L. D.; Popov, I.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 18237. |
| [14] | Liu, S.-L.; Li, X.-H.; Shi, T.-H.; Yang, G.-C.; Wang, H.-L.; Gong, J.-F.; Song, M.- P. Eur. J. Org. Chem. 2017, 2017, 2280. |
| [15] | (a) Li, Y.; Liu, Y.-J.; Shi, B.-F. Adv. Synth. Catal. 2017, 359, 4117. |
| [15] | (b) Rao, W.-H.; Shi, B.-F. Org. Lett. 2015, 17, 2784. |
| [15] | (c) Chen, F.-J.; Liao, G.; Li, X.; Wu, J.; Shi, B.-F. Org. Lett. 2014, 16, 5644. |
| [16] | (a) Jiang, Y.; Feng, Y.-Y.; Zou, J.-X.; Lei, S.; Hu, X.-L.; Yin, G.-F.; Tan, W.; Wang, Z. J. Org. Chem. 2019, 84, 10490. |
| [16] | (b) Kong, W.-J.; Shao, Q.; Li, M.-H.; Zhou, Z.-L.; Xu, H.; Dai, H.-X.; Yu, J.-Q. Organometallics 2018, 37, 2832. |
| [16] | (c) Yan, X.-B.; Gao, P.; Yang, H.-B.; Li, Y.-X.; Liu, X.-Y.; Liang, Y.-M. Tetrahedron 2014, 70, 8730. |
| [17] | (a) Wang, T.; Xu, K.; Zhang, A.; Wang, W.; Liu, L. Chin. J. Org. Chem. 2018, 38, 259. (in Chinese) |
| [17] | (王涛, 许凯, 张安安, 王万里, 刘澜涛, 有机化学, 2018, 38, 259.) |
| [17] | (b) Wang, T.; Xu, K.; Liu, L.; Xie, H.; Li, Y.; Zhao, W-. X. Transition Met. Chem. 2016, 41, 525. |
| [18] | Ouyang, K.; Xi, Z. Acta Chim. Sinica 2013, 71, 13. (in Chinese) |
| [18] | (欧阳昆冰, 席振峰, 化学学报, 2013, 71, 13.) |
| [19] | Shang, M.; Sun, S.-Z.; Dai, H.-X.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 3354. |
/
| 〈 |
|
〉 |