

Chinese Journal of Organic Chemistry >
Copper-Catalyzed C—H Bond and N—H Bond Insertion Reaction Based on Azide-Ynamide Cyclization
Received date: 2020-09-08
Revised date: 2020-10-02
Online published: 2020-10-28
Supported by
National Natural Science Foundation of China(21772161)
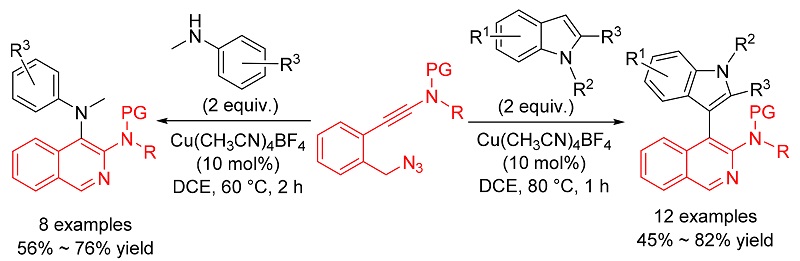
A copper-catalyzed azide-ynamide cyclization to synthesize isoquinoline derivatives is reported. First, α-imino copper carbene intermediate is generated via Cu(I)-catalyzed azide-ynamide cyclization, then this copper carbene can be captured by indoles and anilines to form C—H and N—H insertion products. The notable advantages of this method include a simple procedure, mild reaction conditions and widespread availability of the substrates. Thus, this protocol provides a highly convenient and efficient route for the preparation of natural products and active molecules which contain the isoquinoline-indole or isoquinoline-aniline skeletons.
Key words: cyclization reaction; copper catalysis; copper carbenes; ynamides
Xiaotao Liu , Xin Liu , Longwu Ye . Copper-Catalyzed C—H Bond and N—H Bond Insertion Reaction Based on Azide-Ynamide Cyclization[J]. Chinese Journal of Organic Chemistry, 2021 , 41(3) : 1207 -1215 . DOI: 10.6023/cjoc202009020
| [1] | For selected reviews, see: (a) Khan, A. Y.; Suresh Kumar, G. Biophys. Rev. 2015, 7, 407. |
| [1] | (b) Iranshahy, M.; Quinn, R. J.; Iranshahi, M. RSC Adv. 2014, 4, 15900. |
| [1] | (c) Bentley, K. W. Nat. Prod. Rep. 2006, 23, 444. |
| [1] | (d) Bentley, K. W. Nat. Prod. Rep. 2005, 22, 249. |
| [1] | (e) Bentley, K. W. Nat. Prod. Rep. 2004, 21, 395. |
| [1] | (f) Bentley, K. W. The Isoquinoline Alkaloids, Hardwood Academic, Amsterdam, 1998, Vol. 1. |
| [1] | (g) Phillipson, J. D.; Roberts, M. F.; Zenk, M. H. The Chemistry and Biology of Isoquinoline Alkaloids, Springer Verlag, Berlin, 1985. |
| [2] | (a) Wang, S.-B.; Wang, X.-F.; Qin, B.; Ohkoshi, E.; Hsieh, K.-Y.; Hamel, E.; Cui, M.-T.; Zhu, D.-Q.; Goto, M.; Morris-Natschke, S. L.; Lee, K.-H.; Xie, L. Bioorg. Med. Chem. 2015, 23, 5740. |
| [2] | (b) Smith, N. D.; Bonnefous, C.; Zhuang, H.; Chen, X.; Duron, S.; Lindstrom, A. WO 2009029617, 2009. |
| [2] | (c) Arrington, K. L.; Dudkin, V. Y.; Fraley, M. E.; Garbaccio, R. M.; Hartman, G. D.; Huang, S. Y.; Kreatsoulas, C.; Tasber, E. S. WO 2007008502, 2007. |
| [2] | (d) Allen, J. R.; Amegadzie, A. K.; Gardinier, K. M.; Gregory, G. S.; Hitchcock, S. A.; Hoogestraat, P. J.; Jones, W. D. Jr; Smith, D. L. WO 2005066126, 2005. |
| [2] | (e) Hopper, A.; Schumacher, R. A.; Tehim, A.; De Vivo, M.; Brubaker, W. F. Jr.; Liu, R.; Hess, H.-J. E.; Unterbeck, A. WO 2002074726, 2002. . |
| [3] | For recent selected reviews on the generation of metal carbenes from alkynes, see: (a) Ye, L.-W.; Zhu, X.-Q.; Sahani, R. L.; Xu, Y.; Qian, P.-C.; Liu, R.-S. Chem. Rev. 2021, 121, DOI: 10.1021/acs.chemrev.0c00348. |
| [3] | (b) Sahani, R. L.; Ye, L.-W.; Liu, R.-S. Adv. Organomet. Chem. 2020, 73, 195. |
| [3] | (c) Chen, L.; C hen, K; Zhu, S. Chem 2018, 4, 1208. |
| [3] | Liao, Y.; Zhu, L.; Yu, Y.; Chen, G.; Huang, X. Chin. J. Org. Chem. 2017, 37, 2785. (in Chinese) |
| [3] | (廖云, 朱磊, 俞颖华, 陈贵, 黄学良, 有机化学, 2017, 37, 2785.) |
| [3] | (e) Zi, W.; Toste, F. D. Chem. Soc. Rev. 2016, 45, 4567. |
| [3] | (f) Harris, R. J.; Widenhoefer, R. A. Chem. Soc. Rev. 2016, 45, 4533. |
| [3] | (g) Zheng, Z.; Wang, Z.; Wang, Y.; Zhang, L. Chem. Soc. Rev. 2016, 45, 4448. |
| [3] | (h) Jia, M.; Ma, S. Angew. Chem., Int. Ed. 2016, 55, 9134. |
| [3] | (i) Dorel, R.; Echavarren, A. M. Chem. Rev. 2015, 115, 9028. |
| [3] | (j) Wang, Y.; Muratore, M. E.; Echavarren, A. M. Chem.-Eur. J. 2015, 21, 7332. |
| [3] | (k) Qian, D.; Zhang, J. Chem. Soc. Rev. 2015, 44, 667. |
| [3] | (l) Zhang, L. Acc. Chem. Res. 2014, 47, 877. |
| [4] | For reviews, see: (a) Aguilar, E.; Santamaría, J. Org. Chem. Front. 2019, 6, 1513. |
| [4] | (b) Davies, P. W.; Garzón, M. Asian J. Org. Chem. 2015, 4, 694. |
| [5] | For selected examples, see: (a) Zhu, X.-Q.; Wang, Z.-S.; Hou, B.-S.; Zhang, H.-W.; Deng, C.; Ye, L.-W. Angew. Chem., Int. Ed. 2020, 59, 1666. |
| [5] | (b) Tian, X.; Song, L.; Farshadfar, K.; Rudolph, M.; Rominger, F.; Oeser, T.; Ariafard, A.; Hashmi, A. S. K. Angew. Chem., Int. Ed. 2020, 59, 471. |
| [5] | (c) Zeng, Z.; Jin, H.; Sekine, K.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Angew. Chem., Int. Ed. 2018, 57, 6935. |
| [5] | (d) Sahani, R. L.; Liu, R.-S. Angew. Chem., Int. Ed. 2017, 56, 12736. |
| [5] | (e) Sahani, R. L.; Liu, R.-S. Angew. Chem., Int. Ed. 2017, 56, 1026. |
| [5] | (f) Shen, W.-B.; Xiao, X.-Y.; Sun, Q.; Zhou, B.; Zhu, X.-Q.; Yan, J.-Z.; Lu, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2017, 56, 605. |
| [5] | (g) Zhou, A.-H.; He, Q.; Shu, C.; Yu, Y.-F.; Liu, S.; Zhao, T.; Zhang, W.; Lu, X.; Ye, L.-W. Chem. Sci. 2015, 6, 1265. |
| [6] | For selected examples, see: (a) Garzón, M.; Davies, P. W. Org. Lett. 2014, 16, 4850. |
| [6] | (b) Chatzopoulou, E.; Davies, P. W. Chem. Commun. 2013, 49, 8617. |
| [6] | (c) Davies, P. W.; Cremonesi, A.; Dumitrescu, L. Angew. Chem., Int. Ed. 2011, 50, 8931. |
| [6] | (d) Li, C.; Zhang, L. Org. Lett. 2011, 13, 1738. |
| [7] | For a review, see: Tian, X.; Song, L; Hashmi, A. S. K Chem.-Eur. J. 2020, 26, 3197. |
| [8] | For selected examples, see: (a) Kawada, Y.; Ohmura, S.; Kobayashi, M.; Nojo, W.; Kondo, M.; Matsuda, Y.; Matsuoka, J.; Inuki, S.; Oishi, S.; Wang, C.; Saito, T.; Uchiyama, M.; Suzuki, T.; Ohno, H. Chem. Sci. 2018, 9, 8416. |
| [8] | (b) Lonca, G. H.; Tejo, C.; Chan, H. L.; Chiba, S.; Gagosz, F. Chem. Commun. 2017, 53, 736. |
| [8] | (c) Matsuoka, J.; Matsuda, Y.; Kawada, Y.; Oishi, S.; Ohno, H. Angew. Chem., Int. Ed. 2017, 56, 7444. |
| [8] | (d) Yan, Z.-Y.; Xiao, Y.; Zhang, L. Angew. Chem., Int. Ed. 2012, 51, 8624. |
| [8] | (e) Wetzel, A.; Gagosz, F. Angew. Chem.. Int. Ed. 2011, 50, 7354. |
| [8] | (f) Lu, B.; Luo, Y.; Liu, L.; Ye, L.; Wang, Y.; Zhang, L. Angew. Chem., Int. Ed. 2011, 50, 8358. |
| [8] | (g) Gorin, D. J.; Davis, N. R.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 11260. |
| [9] | For recent reviews on ynamide reactivity, see: (a) Hong, F.-L.; Ye, L.-W. Acc. Chem. Res. 2020, 53, 2003. |
| [9] | (b) Zhou, B.; Tan, T.-D.; Zhu, X.-Q.; Shang, M.; Ye, L.-W. ACS Catal. 2019, 9, 6393. |
| [9] | (c) Pan, F.; Shu, C.; Ye, L.-W. Org. Biomol. Chem. 2016, 14, 9456. |
| [9] | (d) Evano, G.; Theunissen, C.; Lecomte, M. Aldrichim. Acta 2015, 48, 59. |
| [9] | (e) Wang, X.-N.; Yeom, H.-S.; Fang, L.-C.; He, S.; Ma, Z.-X.; Kedrowski, B. L.; Hsung, R. P. Acc. Chem. Res. 2014, 47, 560. |
| [9] | (f) DeKorver, K. A.; Li, H.; Lohse, A. G.; Hayashi, R.; Lu, Z.; Zhang, Y.; Hsung, R. P. Chem. Rev. 2010, 110, 5064. |
| [9] | (g) Evano, G.; Coste, A.; Jouvin, K. Angew. Chem., Int. Ed. 2010, 49, 2840. |
| [10] | For selected examples by our group, see: (a) Liu, X.; Wang, Z.-S.; Zhai, T.-Y.; Luo, C.; Zhang, Y.-P.; Chen, Y.-B.; Deng, C.; Liu, R.-S.; Ye, L.-W. Angew. Chem., Int. Ed. 2020, 59, 17984. |
| [10] | (b) Hong, F.-L.; Chen, Y.-B.; Ye, S.-H.; Zhu, G.-Y.; Zhu, X.-Q.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2020, 142, 7618. |
| [10] | (c) Wang, Z.-S.; Chen, Y.-B.; Zhang, H.-W.; Sun, Z.; Zhu, C.; Ye, L.-W. J. Am. Chem. Soc. 2020, 142, 3636. |
| [10] | (d) Huang, E.-H.; Zhang, Z.-X.; Ye, S.-H.; Chen, Y.-B.; Luo, W.-F.; Qian, P.-C.; Ye, L.-W. Chin. J. Chem. 2020, 38, 1086. |
| [10] | (e) Li, H.-H.; Ye, S.-H.; Chen, Y.-B.; Luo, W.-F.; Qian, P.-C.; Ye, L.-W. Chin. J. Chem. 2020, 38, 263. |
| [10] | (f) Hong, F.-L.; Wang, Z.-S.; Wei, D.-D.; Zhai, T.-Y.; Deng, G.-C.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2019, 141, 16961. |
| [10] | (g) Xu, Y.; Sun, Q.; Tan, T.-D.; Yang, M.-Y.; Yuan, P.; Wu, S.-Q.; Lu, X.; Hong, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2019, 58, 16252. |
| [10] | (h) Zhou, B.; Zhang, Y.-Q.; Zhang, K.; Yang, M.-Y.; Chen, Y.-B.; Li, Y.; Peng, Q.; Zhu, S.-F.; Zhou, Q.-L.; Ye, L.-W. Nat. Commun. 2019, 10, 3234. |
| [10] | (i) Li, L.; Zhu, X.-Q.; Zhang, Y.-Q.; Bu, H.-Z.; Yuan, P.; Chen, J.; Su, J.; Deng, X.; Ye, L.-W. Chem. Sci. 2019, 10, 3123. |
| [10] | (j) Zheng, R.-H.; Guo, H.-C.; Yang, M.-Y.; Liu, M.-Q.; Ye, L.-W. Chin. J. Org. Chem. 2019, 39, 1672. (in Chinese) |
| [10] | (郑人华, 郭海昌, 阳明洋, 刘梦琪, 叶龙武, 有机化学, 2019, 39, 1672.) |
| [10] | (k) Zhu, J.; Ren, X.; Tang, F.; Pan, F.; Ye, L.-W. Chin. J. Org. Chem. 2019, 39, 1102. (in Chinese) |
| [10] | (朱建荣, 任小娟, 唐飞宇, 潘飞, 叶龙武, 有机化学, 2019, 39, 1102.) |
| [10] | (l) Zhou, B.; Li, L.; Zhu, X.-Q.; Yan, J.-Z.; Guo, Y.-L.; Ye, L.-W. Angew. Chem., Int. Ed. 2017, 56, 4015. |
| [10] | (m) Shen, W.-B.; Sun, Q.; Li, L.; Liu, X.; Zhou, B.; Yan, J.-Z.; Lu, X.; Ye, L.-W. Nat. Commun. 2017, 8, 1748. |
| [10] | (n) Li, L.; Chen, X.-M.; Wang, Z.-S.; Zhou, B.; Liu, X.; Lu, X.; Ye, L.-W. ACS Catal. 2017, 7, 4004. |
| [11] | Feng, J.; Yi, X.; Fu, Y.; Yu, Y.; Huang, F. Chin. J. Org. Chem. 2019, 39, 3013. (in Chinese) |
| [11] | (封佳俊, 易享炎, 傅耀锋, 于杨, 黄菲, 有机化学, 2019, 39, 3013.) |
| [12] | Shu, C.; Wang, Y.-H.; Zhou, B.; Li, X.-L.; Ping, Y.-F.; Lu, X.; Ye, L.-W. J. Am. Chem. Soc. 2015, 137, 9567. |
| [13] | Badigenchala, S.; Rajeshkumar, V.; Sekar, G. Org. Biomol. Chem. 2016, 14, 2297. |
/
| 〈 |
|
〉 |