

Chinese Journal of Organic Chemistry >
Syntheses of Chlorophyllous Chlorin Derivatives with Aromatic Ring-Fused Imidazole Structural Unit
Received date: 2020-06-30
Revised date: 2020-10-19
Online published: 2020-11-04
Supported by
National Natural Science Foundations of China(21272048); Natural Science Foundation of Shandong Province(ZR2015BQ012)
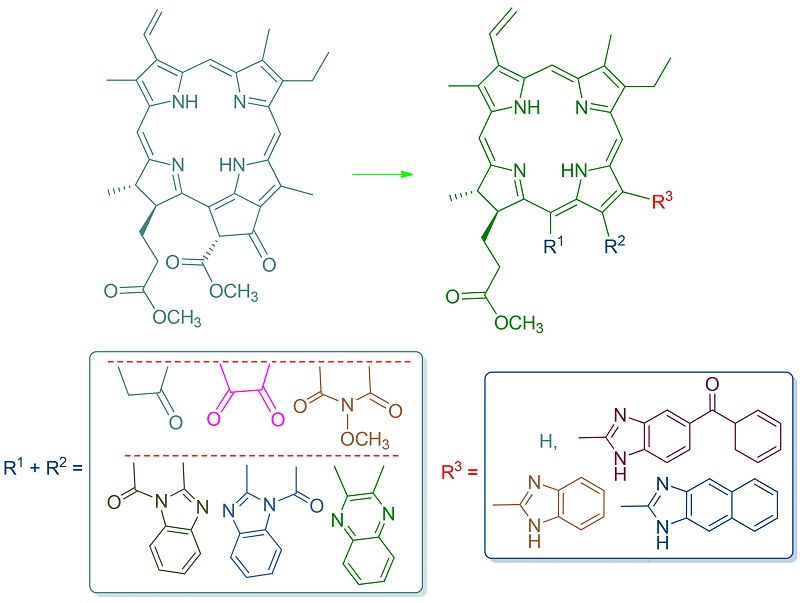
Using pheophorbide-a methyl ester as starting material, the formyl group and α-diketone moiety were introduced into the water-soluble end of N21-N23axis at different positions by the structural transformation on exocyclic ring. Furthermore, using the Phillps-Ladenburg-like reaction of active functional groups in chlorophyll chlorins with various aromatic o-diamines, the aromatic ring-fused imidazole structural units were created in linkage or fused manner, and a series of unreported chlorin derivatives that contain aryl-fused imidazol unit were finally synthesized. Besides, the relevant reaction mechanism was analyzed, and the absorption properties of these imidazolized products were discussed. The chemical structures of all newly synthesized compounds were characterized by elemental analysis, MS, UV-Vis, IR and 1H NMR spectra.
Zhu Zhang , Yu Zhao , Xinyue Wang , Jiazhu Li , Jinjun Wang . Syntheses of Chlorophyllous Chlorin Derivatives with Aromatic Ring-Fused Imidazole Structural Unit[J]. Chinese Journal of Organic Chemistry, 2021 , 41(3) : 1177 -1186 . DOI: 10.6023/cjoc202006080
| [1] | (a) Ding, Y.; Zhu, W.-H.; Xie, Y. Chem. Rev. 2017, 117, 2203. |
| [1] | (b) Li, M.; Wei, P.; Ishida, M.; Li, X.; Savage, M.; Guo, R. Angew. Chem. Int. Ed. 2016, 55, 3063. |
| [1] | (c) Ding, Y.; Tang, Y.; Zhu, W.; Xie, Y. Chem. Soc. Rev. 2015, 44, 1101. |
| [1] | (d) Xie, Y.; Tang, Y.; Wu, W.; Wang, Y.; Liu, J.; Li, X. J. Am. Chem. Soc. 2015, 137, 14055. |
| [2] | (a) St?pień, M.; Gońka, E.; ?y?a, M.; Sprutta, N. Chem. Rev. 2017, 117, 3479. |
| [2] | (b) Ono, N.; Yamada, H.; Okujima, T. In Handbook of Porphyrin Science, Eds.: Kadish, K. M.; Smith, K.M.; Guilard, R., Vol.2. World Scientific Publishing Company, Singapore, 2012, pp.1-102. |
| [2] | (c) Mori, H.; Tanaka, T.; Osuka, A. J. Mater. Chem. C 2013, 1, 2500. |
| [3] | Senge, M. J. Photochem. Photobiol. 1992,3. |
| [4] | (a) Zhang, Z.; Jiang, Q-Y.; Zhang, Q.; Wu, J.; Wang, J.-J. Chin. J. Org. Chem. 2015, 35, 1929. (in Chinese) |
| [4] | (张珠, 姜齐永, 张千, 武进, 王进军, 有机化学, 2015, 35, 1929.) |
| [4] | (b) Li, J.-Z.; He, N.-L.; Liu, Y.; Gai, Y.-Y.; Liu., Y.-M.; Yin, J.-G.; Wang, J.-J. Dyes Pigm. 2017, 146, 189. |
| [4] | (c) Wu, X.-R.; Liu, C.; Yang, Z.; Yao, N.-N.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 632. (in Chinese) |
| [4] | (邬旭然, 刘超, 杨泽, 姚楠楠, 王进军, 有机化学, 2012, 32, 632.) |
| [4] | (d) Zhang, S.-G.; Li, J.-Z.; Zhang, P.; Qi, C.-X.; Wang, J.-J. Chin. J. Org. Chem. 2015, 35, 1060. (in Chinese) |
| [4] | (张善国, 李家柱, 张朋, 祁彩霞, 王进军, 有机化学, 2015, 35, 1060.) |
| [4] | (e) Zhang, Z.; Xu, X.-S.; Li, Y.-L.; Li, J.-Z.; Wang, J.-J. Chin. J. Org. Chem. 2018, 38, 2993. (in Chinese) |
| [4] | (张珠, 徐希森, 李彦龙, 李家柱, 王进军, 有机化学, 2018, 38, 2993.) |
| [4] | (f) Wang, J.-J.; Li, J.-Z.; Jakus, J.; Shim, Y. K. J. Porphyrins Phthalocyanines 2010, 14, 860. |
| [5] | (a) Takahashi, T.; Ogasawara, S.; Shinozaki, Y.; Tamiaki, H. Bull. Chem. Soc. Jpn. 2020, 93, 467. |
| [5] | (b) Kozyrev, A. N.; Suresh, V.; Das, S.; Senge, M. O.; Shibata, M.; Doughertya, T. J.; Pandeya, R. K. Tetrahedron 2000, 56, 3353. |
| [5] | (c) Chen, Y.; Sajjad, M.; Wang, Y..; Batt, C.; Nabi, H. A.; Pandey, R. K. ACS Med. Chem. Lett. 2011, 2, 136. |
| [6] | Mysliwiec, D.; Donnio, B.; Chmielewski, P. J.; Heinrich, B.; Stepien, M. J. Am. Chem. Soc. 2012, 134:4822. |
| [7] | Tokuji, S.; Takahashi, Y.; Shinmori, H.; Shinokubo, H.; Osuka, A. Chem. Commun. 2009,1028. |
| [8] | Akita, M.; Hiroto, S.; Shinokubo, H. Angew. Chem. Int. Ed. 2012, 51, 2894. |
| [9] | Smith, K. M.; Gogg, D. A.; Simpson, D. J. J. Am. Chem. Soc. 1985, 107, 4946. |
| [10] | Wang, J.-J.; Han, G.-F.; Shim, Y.-K. J. Iran. Chem. Soc. 2011, 8, 965. |
| [11] | (a) Wang, J.-J.; Wang, P.; Li, J.-Z.; Jakus, J.; Shin, Y.-K. Bull. Korean Chem. Soc. 2011, 32, 3473. |
| [11] | (b) Wang, P.; Yang, Z.; Li, J.-Z.; Yao, N.-N.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 368. (in Chinese) |
| [11] | (王朋, 杨泽, 李家柱, 姚楠楠, 王进军, 有机化学, 2012, 32, 368.) |
| [11] | (c) Zhang, S.-G. M.S. Thesis, Yantai University, Yantai, 2015. (in Chinese) |
| [11] | (张善国, 硕士论文, 烟台大学, 烟台, 2015.) |
| [11] | (d) Li, J.-Z.; Liu, W.-H.; Li, F.-G.; Wang, J.-J.; Suo, Y.-R.; Liu, Y.-J. Chin. J. Org. Chem. 2007, 27, 1594. (in Chinese) |
| [11] | (李家柱, 刘万卉, 李付国, 王进军, 索有瑞, 刘永军, 有机化学, 2007, 27, 1594.) |
| [11] | (e) liu, R.-R.; Wang, L.-M.; Yin, J.-G.; Wu, J.; Liu, C.; Zhang, P.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 318. (in Chinese) |
| [11] | (刘冉冉, 王鲁敏, 殷军港, 武进, 刘超, 张朋, 王进军, 有机化学, 2012, 32, 318.) |
| [12] | Wang, J.-J.; Shim, Y.-K.; Jiang, G.-J.; Imafuku, K. J. Heterocycl. Chem. 2003, 40, 1075. |
| [13] | Tamiaki, H.; Wada, A.; Matsubara, S. J. Photochem. Photobiol. A: Chem. 2018, 353, 581. |
| [14] | Matsubara, S.; Shoji, S.; Tamiaki, H. J. Photochem. Photobiol. A: Chem. 2017, 340, 53. |
| [15] | (a) Pandey, S. K.; Zheng, X.; Morgan, J.; Missert, J. R.; Liu, T.-H.; Shibata, M.; Bellnier, D. A.; Oseroff, A. R.; Henderson, B. W.; Dougherty, T. J.; Pandey, R. K. Mol. Pharm. 2007, 4, 448. |
| [15] | (b) Li, J.; He, N.; Liu, Y.; Zhang, Z.; Zhang, X.; Han, X.; Gai, Y.; Liu, Y.; Yin, J.; Wang, J. Dyes Pigments. 2017, 146, 189. |
| [15] | (c) Jiang, Q.-Y.; Zhang, Z.; Liu, Y.; Yao, N.-N.; Wang, J-J. Chin. J. Org. Chem. 2017, 37, 1814. (in Chinese) |
| [15] | (姜齐永, 张珠, 刘洋, 姚楠楠, 王进军, 有机化学, 2017, 37, 1814.) |
| [15] | (d) Duan, S.; Dall'Agnese, C.; Chen, G.; Wang, X.-F.; Tamiaki, H.; Yamamoto, Y.; Ikeuchi, T.; Sasaki, S. ACS Energy Lett. 2018, 3, 1708. |
| [15] | Wang, J.-J. Chin. J. Org. Chem. 2005, 25, 1353. (in Chinese) |
| [15] | (王进军, 有机化学, 2005, 25, 1353.) |
| [16] | Liu, H.-Y.; Zhu, G.-H.; Liu, R.-R.; Jin, Y.-X.; Qi, C-X.; Wang, J.-J. Chin. J. Org. Chem. 2015, 35, 1320. (in Chinese) |
| [16] | (刘红瑶, 朱国华, 刘冉冉, 金英学, 祁彩霞, 王进军, 有机化学, 2015, 35, 1320.) |
/
| 〈 |
|
〉 |