

Chinese Journal of Organic Chemistry >
Synthesis of Nopinone-Based Quinazolin-2-amine Fluorescent Probe for Detection of Cu2+ and Its Application Research
Received date: 2020-08-26
Revised date: 2020-10-22
Online published: 2020-11-04
Supported by
National Natural Science Foundation of China(32071707); Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-Forest Biomass, and the Priority Academic Program Development of Jiangsu Higher Education Institutions
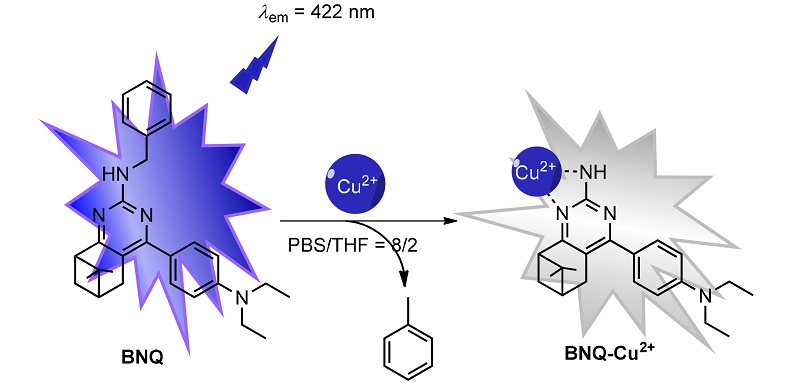
In this study, a novel nopinone-based quinazolin-2-amine fluorescent probe N-benzyl-4-(4-(diethylamino)phenyl)- 7,7-dimethyl-5,6,7,8-tetrahydro-6,8-methanoquinazolin-2-amine (BNQ) was synthesized from renewable β-pinene derivative nopinone. The probe BNQ exhibited a high selective fluorescence quenching response toward Cu2+in PBS/THF (V/V=8/2, 10 mmol?L–1, pH=7.4) solution, and could effectively recognize Cu2+ within a wide pH range (4~10). Meanwhile, the fluorescent titration experiment showed that the probe BNQ was high sensitive for detecting Cu2+, and its detection limit was found to be 0.09 μmol?L –1, which was much lower than that of stated by World Health Organization (WHO) in drinking water (<30 μmol?L–1). Furthermore, the coordination mechanism of probe BNQ with Cu2+ was confirmed by high resolution mass spectrum (HRMS) and density functional theory (DFT) calculations. The probe BNQ could detect micromolar Cu2+ in different environmental water samples. The results of bio-imaging experiment demonstrated that the probe BNQwas also successfully used for monitoring Cu2+ in living zebrafish, and showed an excellent fluorescence imaging performance.
Key words: nopinone; quinazolin-2-amine; fluorescent probe; Cu2+ ion; bioimaging
Mingguang Zhang , Mingxin Li , Yiqin Yang , Xu Xu , Jie Song , Zhonglong Wang , Shifa Wang . Synthesis of Nopinone-Based Quinazolin-2-amine Fluorescent Probe for Detection of Cu2+ and Its Application Research[J]. Chinese Journal of Organic Chemistry, 2021 , 41(3) : 1168 -1176 . DOI: 10.6023/cjoc202008049
| [1] | Chen, S. H.; Pang, C. M.; Chen, X. Y.; Yan, Z. H.; Huang, S. M.; Li, X. D.; Zhong, Y. T.; Wang, C. Y. Chin. J. Org. Chem. 2019, 39, 1846. (in Chinese) |
| [1] | (陈思鸿, 庞楚明, 陈孝云, 严智浩, 黄诗敏, 李香弟, 钟雅婷, 汪朝阳, 有机化学, 2019, 39, 1846.) |
| [2] | Yue, Y.; Huo, F.; Cheng, F. Chem. Soc. Rev. 2019, 48, 4155. |
| [3] | Ma, C.; Zhong, G.; Zhao, Y. Spectrochim. Acta, Part A 2020,118545. |
| [4] | Singh, H.; Tiwari, K.; Tiwari, R. Chem. Rev. 2019, 119, 11718. |
| [5] | Chen, X.; Pradhan, T.; Wang, F. Chem. Rev. 2012, 112, 1910. |
| [6] | Cao, D.; Liu, Z.; Verwilst, P. Chem. Rev. 2019, 119, 10403. |
| [7] | Shi, Y.; Yu, Y. W.; Xue, L.; Wang, Y. F. Chin. J. Org. Chem. 2019, 39, 3414. (in Chinese) |
| [7] | (石岩, 于有伟, 薛林, 王延风, 有机化学, 2019, 39, 3414.) |
| [8] | Zhao, Y.; Luo, Y.; Wang, H. Anal. Chim. Acta 2019, 1065, 134. |
| [9] | Patil, A.; Salunke, G. S. Inorg. Chim. Acta 2018, 482, 99. |
| [10] | Han, Z.; Nan, D.; Yang, H. Sens. Actuators, B 2019, 298, 126842. |
| [11] | Murugan, N.; Prakash, M.; Jayakumar, M. Appl. Surf. Sci. 2019, 476, 468. |
| [12] | Zhang, Y.; Li, H.; Pu, S. J. Photochem. Photobiol., A 2020,112721. |
| [13] | Wang, R.; Zhang, L.; Liu, R. Carbohydr. Polym. 2019, 223, 115117. |
| [14] | Meng, X. J.; Zhao, J. Z.; Ma, W. B. Chin. J. Org. Chem. 2020, 40, 276. (in Chinese) |
| [14] | (孟宪娇, 赵晋忠, 马文兵, 有机化学, 2020, 40, 276.) |
| [15] | Que, E. L.; Domaille, D. W.; Chang, C. J. Chem. Rev. 2008, 108, 1517. |
| [16] | Mathie, A.; Sutton, G. L.; Clarke, C. E. Pharmacol. Ther. 2006, 111, 567. |
| [17] | Georgopoulos, A.; Yonone, L. M.; Opiekun, R. E. J. Toxicol. Environ. Health, Part B 2001, 4, 341. |
| [18] | Barnham, K.; Masters, C.; Bush, A. Nat. Rev. Drug Discovery 2004, 3, 205. |
| [19] | Meng, Q.; Shi, Y.; Wang, C. Org. Biomol. Chem. 2015, 13, 2918. |
| [20] | Chen, D.; Chen, P.; Zong, L. R. Soc. Open Sci. 2017, 4, 171161. |
| [21] | Chen, F.; Hou, F.; Huang, L. Dyes Pigm. 2013, 98, 146. |
| [22] | Gaggelli, E.; Kozlowski, H.; Valensin, D. Chem. Rev. 2006, 106, 1995. |
| [23] | Dell'Acqua, S.; Pirota, V.; Anzani, C.; Rocco, M. M.; Nicolis, S.; Valensin, D.; Monzani, E.; Casella, L. Metallomics 2015, 7, 1091. |
| [24] | World Health Organization Guidelines for Drinking-Water Quality, 2004. |
| [25] | Jonas, R. Appl. Environ. Microbiol. 1989, 55, 43. |
| [26] | Xiao, Q.; Gao, H.; Yuan, Q. J. Chromatogr., A 2013, 1274, 145. |
| [27] | Wang, X.; Luo, C.; Li, L. J. Electroanal. Chem. 2015, 757, 100. |
| [28] | Selvarajan, S.; Alluri, N.; Chandrasekhar, R. Biosensors Bioelectron. 2017, 91, 203. |
| [29] | Ahmed, K.; Sengan; Veerappan, A. Sens. Actuators, B 2016, 233, 431. |
| [30] | Li, Y.; Zhou, H.; Yin, S. Sens. Actuators, B 2016, 235, 33. |
| [31] | He, C.; Zhou, H.; Yang, N. New J. Chem. 2018, 42, 2520. |
| [32] | Duan, G.; Zhang, G.; Yuan, S. Spectrochim. Acta, Part A 2019, 219, 173. |
| [33] | Zheng, X.; Ji, R.; Cao, X. Anal. Chim. Acta 2017, 978, 48. |
| [34] | Wang, Y.; Liu, S.; Chen, H. Dyes Pigm. 2017, 142, 293. |
| [35] | Liu, S.; Liu, Y.; Pan, H. Tetrahedron Lett. 2.018, 59, 108. |
| [36] | Feng, Y.; Yang, Y.; Wang, Y. Sens. Actuators, B 2019, 288, 27. |
| [37] | Yin, G.; Yao, J.; Hong, S. Analyst 2019, 144, 6962. |
| [38] | Zhang, X.; Sun, P.; Li, F. Sens. Actuators, B 2018, 255, 366. |
| [39] | Xu, Z.; Wang, H.; Chen, Z. Spectrochim. Acta, Part A 2019, 216, 404. |
| [40] | Zhu, D.; Luo, Y.; Shuai, L. Tetrahedron Lett. 2016, 57, 5326. |
| [41] | Choi, M.; Kim, G.; Hong, J. Tetrahedron Lett. 2016, 57, 975. |
| [42] | Wang, Y.; Zhou, J.; Zhao, L. Dyes Pigm. 2020,108513. |
| [43] | Huang, C.; Li, H.; Luo, Y. Dalton Trans. 2014, 43, 8102. |
| [44] | Shen, R.; Yang, J.; Luo, H. Tetrahedron 2017, 73, 373. |
| [45] | Liu, Y.; Yang, L.; Li, P. Spectrochim. Acta, Part A 2020, 227, 117540. |
| [46] | Peng, L.; Zhao, Q.; Wang, D. Sens. Actuators, B 2009, 136, 80. |
| [47] | Jiang, Q.; Wang, Z., Li, M. Dyes Pigm. 2019, 171, 107702. |
/
| 〈 |
|
〉 |