

Chinese Journal of Organic Chemistry >
Recent Advances in the Ring-Forming Reactions of Ynamides
Received date: 2020-09-09
Revised date: 2020-10-21
Online published: 2020-11-19
Supported by
National Natural Science Foundation of China(81803406); National Natural Science Foundation of China(U1804283); Foundation of Henan Educational Committee(19A150009)
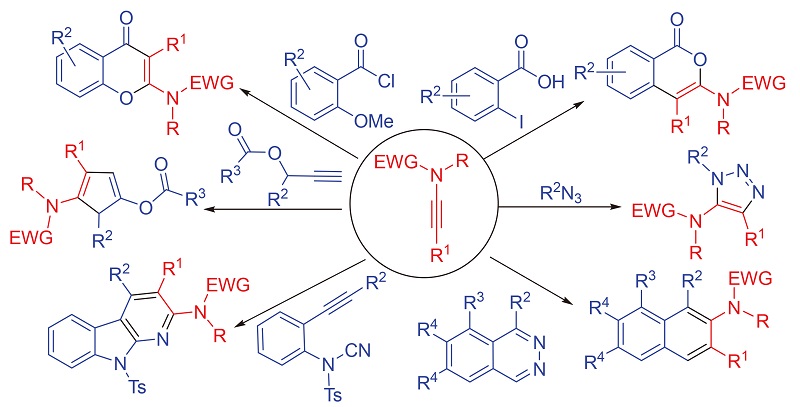
As a subgroup of heteroatom-substituted alkynes, ynamide has unique structural characteristics. The alkynyl group of ynamide is activated by the conjugation of nitrogen lone pair, at the same time by simply placing an electron-withdrawing group on the nitrogen atom, the donating ability of the nitrogen lone pair toward the alkynyl motif is greatly diminished through resonance delocalization into the electron-withdrawing group. Consequently, ynamides have set the gold standard for balancing reactivity and stability, and have become highly versatile organic synthons applicable to a diverse array of transformations that can be useful for natural product syntheses. Especially with the emergence of efficient and atom-economical preparation methods, the field of ynamide chemistry has rapidly expanded. Among the various reported organic transformations involving ynamides, research on ring-forming reactions is the most prevalent. On one hand, this is closely related to the structural characteristic of ynamide (this activated alkyne has both electrophilic and nucleophilic properties, which are conducive to the formation of rings). On the other hand, the reactions of ynamides directly afford nitrogen-containing cyclic compounds, which provide access to important structural entities found in natural products and pharmacophores. These properties have contributed to a dramatic increase in the number of publications over the past few years. This review aims to examine the literatures from late 2010 through early 2020 related to the use of ynamides in ring-forming transformations. And it is organized by the reaction types of ring formation including radical cyclizations, ring-closing metathesis, transition metal and non-transition metal mediated cyclizations, cycloaddition reactions, and rearrangements. However, due to the emergence of a large number of ring-forming reaction involving ynamides, not all the beautiful recent works are presented, and representative examples are selected to demonstrate the scope and mechanistic insight of these ring-forming transformations.
Key words: ynamide; nitrogen heterocycle; cyclization; cycloaddition; rearrangement
Xinyue Zhou , Zongxian Liang , Xiao-Na Wang . Recent Advances in the Ring-Forming Reactions of Ynamides[J]. Chinese Journal of Organic Chemistry, 2021 , 41(4) : 1288 -1318 . DOI: 10.6023/cjoc202009025
| [1] | Viehe, H.G. Angew. Chem., Int. Ed. 1963, 2,477. |
| [2] | Janousek, Z.; Collard, J.; Viehe, H.G. Angew. Chem., Int. Ed. 1972, 11,917. |
| [3] | (a) Evano, G.; Coste, A.; Jouvin, K. Angew. Chem., Int. Ed. 2010, 49,2840. |
| [3] | (b) DeKorver, K.A.; Li, H.; Lohse, A.G.; Hayashi, R.; Lu, Z.; Zhang, Y.; Hsung, R.P. Chem. Rev. 2010, 110,5064. |
| [3] | (c) Wang, X.-N.; Yeom, H.-S.; Fang, L.-C.; He, S.; Ma, Z.-X.; Kedrowski, B.L.; Hsung, R.P. Acc. Chem. Res. 2014, 47,560. |
| [3] | (d) Evano, G.; Theunissen, C.; Lecomte, M. Aldrichim. Acta 2015, 48,59. |
| [3] | (e) Pan, F.; Shu, C.; Ye, L.-W. Org. Biomol. Chem. 2016, 14,9456. |
| [3] | (f) Liao, Y.; Zhu, L.; Yu, Y.; Chen, G.; Huang, X. Chin. J. Org. Chem. 2017, 37,2785. (in Chinese) |
| [3] | ( 廖云, 朱磊, 俞颖华, 陈贵, 黄学良, 有机化学, 2017, 37,2785.) 2578dc51-76c9-4272-b262-bd95d94f25c8 |
| [3] | (g) Zhou, B.; Tan, T.-D.; Zhu, X.-Q.; Shang, M.; Ye, L.-W. ACS Catal. 2019, 9,6393. |
| [3] | (h) Hong, F.-L.; Ye, L.-W. Acc. Chem. Res. 2020, 53,2003. |
| [4] | Balieu, S.; Toutah, K.; Carro, L.; Chamoreau, L.-M.; Rousselière, H.; Courillon, C. Tetrahedron Lett. 2011, 52,2876. fd0395e4-17bb-4cea-b580-bf27d1e41cf9 |
| [5] | Marion, F.; Courillon, C.; Malacria, M. Org. Lett. 2003, 5,5095. |
| [6] | (a) Chemla, F.; Dulong, F.; Ferreira, F.; Nüllen, M.; Pérez-Luna, A. Synthesis 2011,1347. |
| [6] | (b) Romain, E.; Fopp, C.; Chemla, F.; Ferreira, F.; Jackowski, O.; Oestreich, M.; Perez-Luna, A. Angew. Chem., Int. Ed. 2014, 53,11333. |
| [6] | (c) de la Vega-Hernández, K.; Romain, E.; Coffinet, A.; Bijouard, K.; Gontard, G.; Chemla, F.; Ferreira, F.; Jackowski, O.; Perez-Luna, A. J. Am. Chem. Soc. 2018, 140,17632. |
| [7] | Dutta, S.; Mallick, R.K.; Prasad, R.; Gandon, V.; Sahoo, A.K. Angew. Chem., Int. Ed. 2019, 58,2289. |
| [8] | Casse, M.; Nisole, C.; Dossmann, H.; Gimbert, Y.; Fourquez, J.-M.; Haberkorn, L.; Ollivier, C.; Fensterbank, L. Sci. China: Chem. 2019, 62,1542. |
| [9] | Wakamatsu, H.; Sakagami, M.; Hanata, M.; Takeshita, M.; Mori, M. Macromol. Symp. 2010, 293,5. |
| [10] | Wakamatsu, H.; Sasaki, Y.; Kawahata, M.; Yamaguchi, K.; Yoshimura, Y. Synthesis 2018, 50,3467. |
| [11] | Poloukhtine, A.; Rassadin, V.; Kuzmin, A.; Popik, V.V. J. Org. Chem. 2010, 75,5953. |
| [12] | Gati, W.; Rammah, M.M.; Rammah, M.B.; Couty, F.; Evano, G. J. Am. Chem. Soc. 2012, 134,9078. |
| [13] | Meng, T.-J.; Chen, R.-X.; Liu, L.-T.; Wang, T.; Liu, X.-M.; Zhao, W.-X. Chin. J. Org. Chem. 2015, 35,2108. (in Chinese) |
| [13] | ( 孟团结, 陈荣祥, 刘澜涛, 王涛, 刘新明, 赵文献, 有机化学, 2015, 35,2108.) |
| [14] | Kong, Y.; Jiang, K.; Cao, J.; Fu, L.; Yu, L.; Lai, G.; Cui, Y.; Hu, Z.; Wang, G. Org. Lett. 2013, 15,422. |
| [15] | Willumstad, T.P.; Boudreau, P.D.; Danheiser, R.L. J. Org. Chem. 2015, 80,11794. |
| [16] | Liu, H.; Yang, Y.; Wang, S.; Wu, J.; Wang, X.-N.; Chang, J. Org. Lett. 2015, 17,4472. |
| [17] | Lecomte, M.; Evano, G. Angew. Chem., Int. Ed. 2016, 55,4547. |
| [18] | Wang, Y.; Lin, J.; Wang, X; Wang, G.; Zhang, X.; Yao, B.; Zhao, Y.; Yu, P.; Lin, B.; Liu, Y.; Cheng, M. Chem.-Eur. J. 2018, 24,4026. |
| [19] | Brutiu, B.R.; Bubeneck, W.A.; Cvetkovic, O.; Li, J.; Maulide, N. Monatsh. Chem. 2018, 150,3. |
| [20] | Zhang, J.; Li, S.; Qiao, Y.; Peng, C.; Wang, X.-N.; Chang, J. Chem. Commun. 2018, 54,12455. |
| [21] | Yoo, H.J.; Youn, S.W. Org. Lett. 2019, 21,3422. |
| [22] | Li, L.; Zhou, B.; Wang, Y.-H.; Shu, C.; Pan, Y.-F.; Lu, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2015, 54,8245. |
| [23] | Li, H.-H.; Ye, S.-H.; Chen, Y.-B.; Luo, W.-F.; Qian, P.-C.; Ye, L.-W. Chin. J. Chem. 2020, 38,263. |
| [24] | Nishimura, T.; Takiguchi, Y.; Maeda, Y.; Hayashi, T. Adv. Synth. Catal. 2013, 355,1374. |
| [25] | Liao, Y.; Lu, Q.; Chen, G.; Yu, Y.; Li, C.; Huang, X. ACS Catal. 2017, 7,7529. |
| [26] | Okamoto, N.; Yanada, R.; Sueda, T. Eur. J. Org. Chem. 2019,691. |
| [27] | Greenaway, R.L.; Campbell, C.D.; Holton, O.T.; Russell, C.A.; Anderson, E.A. Chem.-Eur. J. 2011, 17,14366. |
| [28] | Greenaway, R.L.; Campbell, C.D.; Chapman, H.A.; Anderson, E.A. Adv. Synth. Catal. 2012, 354,3187. |
| [29] | Cao, J.; Xu, Y.; Kong, Y.; Cui, Y.; Hu, Z.; Wang, G.; Deng, Y.; Lai, G. Org. Lett. 2012, 14,38. |
| [30] | Huang, H.; He, G.; Zhu, G.; Zhu, X.; Qiu, S.; Zhu, H. J. Org. Chem. 2015, 80,3480. |
| [31] | Liu, H.; Yang, Y.; Wu, J.; Wang, X.-N.; Chang, J. Chem. Commun. 2016, 52,6801. |
| [32] | Reddy, A.S.; Kumari, A.L. S.; Swamy, K.C. K. Tetrahedron 2017, 73,2766. |
| [33] | Bhunia, S.; Chang, C.-J.; Liu, R.-S. Org. Lett. 2012, 14,5522. |
| [34] | Shen, W.-B.; Xiao, X.-Y.; Sun, Q.; Zhou, B.; Zhu, X.-Q.; Yan, J.-Z.; Lu, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2017, 56,605. |
| [35] | Hashmi, A.S. K.; Schuster, A.M.; Zimmer, M.; Rominger, F. Chem.-Eur. J. 2011, 17,5511. |
| [36] | Gati, W.; Couty, F.; Boubaker, T.; Rammah, M.M.; Rammah, M.B.; Evano, G. Org. Lett. 2013, 15,3122. |
| [37] | Reddy, A.S.; Reddy, M.N.; Swamy, K.C. K. RSC Adv. 2014, 4,28359. |
| [38] | Nickel, J.; Fernández, M.; Klier, L.; Knochel, P. Chem.-Eur. J. 2016, 22,14397. |
| [39] | Baguia, H.; Deldaele, C.; Romero, E.; Michelet, B.; Evano, G. Synthesis 2018, 50,3022. |
| [40] | Hong, F.-L.; Wang, Z.-S.; Wei, D.-D.; Zhai, T.-Y.; Deng, G.-C.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2019, 141,16961. |
| [41] | Hong, F.-L.; Chen, Y.-B.; Ye, S.-H.; Zhu, G.-Y.; Zhu, X.-Q.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2020, 142,7618. |
| [42] | Liu, X.; Wang, Z.-S.; Zhai, T.-Y.; Luo, C.; Zhang, Y.-P.; Chen, Y.-B.; Deng, C.; Liu, R.-S.; Ye, L.-W. Angew. Chem., Int. Ed. 2020, 59,17984. |
| [43] | Garcia, P.; Harrak, Y.; Diab, L.; Cordier, P.; Ollivier, C.; Gandon, V.; Malacria, M.; Fensterbank, L.; Aubert, C. Org. Lett. 2011, 13,2952. |
| [44] | Liang, G.; Ji, Y.; Liu, H.; Pang, Y.; Zhou, B.; Cheng, M.; Liu, Y.; Lin, B.; Liu, Y. Adv. Synth. Catal. 2020, 362,192. |
| [45] | Ieawsuwan, W.; Ruchirawat, S. Heterocycles 2019, 99,100. |
| [46] | Blanco Jaimes, M.C.; Weingand, V.; Rominger, F.; Hashmi, A.S. K. Chem.-Eur. J. 2013, 19,12504. |
| [47] | Rettenmeier, E.; Schuster, A.M.; Rudolph, M.; Rominger, F.; Gade, C.A.; Hashmi, A.S. K. Angew. Chem., Int. Ed. 2013, 52,5880. |
| [48] | Tokimizu, Y.; Oishi, S.; Fujii, N.; Ohno, H. Org. Lett. 2014, 16,3138. |
| [49] | Tokimizu, Y.; Wieteck, M.; Rudolph, M.; Oishi, S.; Fujii, N.; Hashmi, A.S. K.; Ohno, H. Org. Lett. 2015, 17,604. |
| [50] | Shu, C.; Wang, Y.-H.; Zhou, B.; Li, X.-L.; Ping, Y.-F.; Lü, X.; Ye, L.-W. J. Am. Chem. Soc. 2015, 137,9567. |
| [51] | Shu, C.; Wang, Y.-H.; Shen, C.-H.; Ruan, P.-P.; Lü, X.; Ye, L.-W. Org. Lett. 2016, 18,3254. |
| [52] | Jin, H.; Tian, B.; Song, X.; Xie, J.; Rudolph, M.; Rominger, F.; Hashmi, A.S. K. Angew. Chem., Int. Ed. 2016, 55,12688. |
| [53] | Shen, W.-B.; Sun, Q.; Li, L.; Liu, X.; Zhou, B.; Yan, J.-Z.; Lü, X.; Ye, L.-W. Nat. Commun. 2017, 8,1748. |
| [54] | Ito, M.; Kawasaki, R.; Kanyiva, K.S.; Shibata, T. Chem.-Eur. J. 2018, 24,3721. |
| [55] | Xu, W.; Wang, G.; Xie, X.; Liu, Y. Org. Lett. 2018, 20,3273. |
| [56] | Hsu, Y.-C.; Hsieh, S.-A.; Liu, R.-S. Chem.-Eur. J. 2019, 25,5288. |
| [57] | Vanjari, R.; Dutta, S.; Gogoi, M.P.; Gandon, V.; Sahoo, A.K. Org. Lett. 2018, 20,8077. |
| [58] | Rode, N.D.; Arcadi, A.; Nicola, A.D.; Marinelli, F.; Michelet, V. Org. Lett. 2018, 20,5103. |
| [59] | Febvay, J.; Sanogo, Y.; Retailleau, P.; Gogoi, M.P.; Sahoo, A.K.; Marinetti, A.; Voituriez, A. Org. Lett. 2019, 21,9281. |
| [60] | Li, H.; Hsung, R.P.; Dekorver, K.A.; Wei, Y. Org. Lett. 2010, 12,3780. |
| [61] | Schotes, C.; Mezzetti, A. Angew. Chem., Int. Ed. 2011, 50,3072. |
| [62] | Smith, D.L.; Chidipudi, S.R.; Goundry, W.R.; Lam, H.W. Org. Lett. 2012, 14,4934. |
| [63] | Yuan, Y.; Bai, L.; Nan, J.; Liu, J.; Luan, X. Org. Lett. 2014, 16,4316. |
| [64] | Wang, X.-N.; Ma, Z.-X.; Deng, J.; Hsung, R.P. Tetrahedron Lett. 2015, 56,3463. |
| [65] | Chen, L.; Cao, J.; Xu, Z.; Zheng, Z.-J.; Cui, Y.-M.; Xu, L.-W. Chem. Commun. 2016, 52,9574. |
| [66] | Yang, Y.; Liu, H.; Peng, C.; Wu, J.; Zhang, J.; Qiao, Y.; Wang, X.-N.; Chang, J. Org. Lett. 2016, 18,5022. |
| [67] | Peng, C.; Zhang, J.; Xue, J.; Li, S.; Wang, X.-N.; Chang, J. J. Org. Chem. 2018, 83,9256. |
| [68] | Davies, P.W.; Cremonesi, A.; Dumitrescu, L. Angew. Chem., Int. Ed. 2011, 50,8931. |
| [69] | Mackay, W.D.; Fistikci, M.; Carris, R.M.; Johnson, J.S. Org. Lett. 2014, 16,1626. |
| [70] | (a) Zhou, A.-H.; He, Q.; Shu, C.; Yu, Y.-F.; Liu, S.; Zhao, T.; Zhang, W.; Lü, X.; Ye, L.-W. Chem. Sci. 2015, 6,1265. |
| [70] | (b) Li, X.-L.; Wang, J.-Q.; Li, L.; Yin, Y.-W.; Ye, L.-W. Acta Chim. Sinica 2016, 74,49. (in Chinese) |
| [70] | ( 李新玲, 王佳琪, 李龙, 尹应武, 叶龙武, 化学学报, 2016, 74,49.) |
| [71] | Yu, Y.; Chen, G.; Zhu, L.; Liao, Y.; Wu, Y.; Huang, X. J. Org. Chem. 2016, 81,8142. |
| [72] | Zhao, Y.; Hu, Y.; Wang, C.; Li, X.; Wan, B. J. Org. Chem. 2017, 82,3935. |
| [73] | Zhao, Y.; Hu, Y.; Li, X.; Wan, B. Org. Biomol. Chem. 2017, 15,3413. |
| [74] | Tian, X.; Song, L.; Han, C.; Zhang, C.; Wu, Y.; Rudolph, M.; Rominger, F.; Hashmi, A.S. K. Org. Lett. 2019, 21,2937. |
| [75] | Lin, P.-P.; Han, X.-L.; Ye, G.-H.; Li, J.-L.; Li, Q.; Wang, H. J. Org. Chem. 2019, 84,12966. |
| [76] | Mak, X.Y.; Crombie, A.L.; Danheiser, R.L. J. Org. Chem. 2011, 76,1852. |
| [77] | Pawar, S.K.; Vasu, D.; Liu, R.-S. Adv. Synth. Catal. 2014, 356,2411. |
| [78] | Duret, G.; Quinlan, R.; Martin, R.E.; Bisseret, P.; Neuburger, M.; Gandon, V.; Blanchard, N. Org. Lett. 2016, 18,1610. |
| [79] | Xue, J.; Gao, E.; Wang, X.-N.; Chang, J. Org. Lett. 2018, 20,6055. |
| [80] | Zhao, X.; Song, X.; Jin, H.; Zeng, Z.; Wang, Q.; Rudolph, M.; Rominger, F.; Hashmi, A.S. K. Adv. Synth. Catal. 2018, 360,2720. |
| [81] | Wu, H.; Liu, Y.; He, M.-X.; Wen, H.; Cao, W.; Chen, P.; Tang, Y. Org. Biomol. Chem. 2019, 17,8408. |
| [82] | Nissen, F.; Richard, V.; Alayrac, C.; Witulski, B. Chem. Commun. 2011, 47,6656. |
| [83] | Garcia, P.; Evanno, Y.; George, P.; Sevrin, M.; Ricci, G.; Malacria, M.; Aubert, C.; Gandon, V. Chem.-Eur. J. 2012, 18,4337. |
| [84] | Karad, S.N.; Liu, R.-S. Angew. Chem., Int. Ed. 2014, 53,9072. |
| [85] | Liang, H.; Bi, S.; Liu, Y.; Tang, Y.-N.; Liu, C. Org. Biomol. Chem. 2016, 14,2637. |
| [86] | Liu, D.; Nie, Q.; Cai, M. Tetrahedron 2018, 74,3020. |
| [87] | Chen, P.; Song, C.-X.; Wang, W.-S.; Yu, X.-L.; Tang, Y. RSC Adv. 2016, 6,80055. |
| [88] | Zhang, J.; Zhang, Q.; Xia, B.; Wu, J.; Wang, X.-N.; Chang, J. Org. Lett. 2016, 18,3390. |
| [89] | Wen, H.; Cao, W.; Liu, Y.; Wang, L.; Chen, P.; Tang, Y. J. Org. Chem. 2018, 83,13308. |
| [90] | Zhang, J.; Guo, M.; Chen, Y.; Zhang, S.; Wang, X.-N.; Chang, J. Org. Lett. 2019, 21,1331. |
| [91] | Dateer, R.B.; Pati, K.; Liu, R.-S. Chem. Commun. 2012, 48,7200. |
| [92] | Pawar, S.K.; Sahani, R.L.; Liu, R.-S. Chem.-Eur. J. 2015, 21,10843. |
| [93] | Jadhav, P.D.; Lu, X.; Liu, R.-S. ACS Catal. 2018, 8,9697. |
| [94] | Han, X.-L.; Liu, X.-G.; Lin, E.; Chen, Y.; Chen, Z.; Wang, H.; Li, Q. Chem. Commun. 2018, 54,11562. |
| [95] | Jin, H.; Rudolph, M.; Rominger, F.; Hashmi, A.S. K. ACS Catal. 2019, 9,11663. |
| [96] | Dekorver, K.A.; Hsung, R.P.; Lohse, A.G.; Zhang, Y. Org. Lett. 2010, 12,1840. |
| [97] | Brioche, J.; Meyer, C.; Cossy, J. Org. Lett. 2013, 15,1626. |
| [98] | Zhou, B.; Li, L.; Zhu, X.-Q.; Yan, J.-Z.; Guo, Y.-L.; Ye, L.-W. Angew. Chem., Int. Ed. 2017, 56,4015. |
| [99] | Baker, T.; Davies, P.W. Eur. J. Org. Chem. 2019,5201. |
| [100] | Wang, Z.-S.; Chen, Y.-B.; Zhang, H.-W.; Sun, Z.; Zhu, C.; Ye, L.-W. J. Am. Chem. Soc. 2020, 142,3636. |
/
| 〈 |
|
〉 |