

Chinese Journal of Organic Chemistry >
Design, Synthesis and Activity Evaluation of Novel Bromodomain-Containing Protein 4 (BRD4) Small Molecule Inhibitor Based on ABBV-075
Received date: 2020-07-26
Revised date: 2020-10-21
Online published: 2020-12-05
Supported by
National Natural Science Foundation of China(81438005); National Natural Science Foundation of China(81773562); National Key Research Program of Proteins(SQ2018YFE011359); Key Research Program of Henan Province(1611003110100)
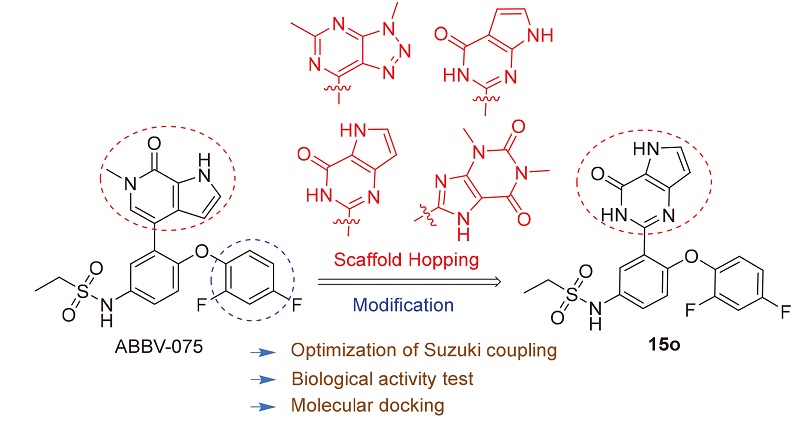
In order to discover novel bromodomain-containing protein 4 (BRD4) small molecule inhibitor, 16 compounds with 4 different nuclei based on ABBV-075 were designed and synthesized through scaffold hopping. The conditions of the Suzuki coupling reaction in the synthesis steps were optimized and the activity of all compounds against BRD4 was tested. The results indicated that compound N-(4-(2,4-difluorophenoxy)-3-(4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidin-2-yl)- phenyl)ethanesulfonamide (15o) had 51% at concentration of 10 μmol/L with IC 50 value of (16.39±1.20) μmol/L, possessing obvious BRD4 inhibitory activity. The molecular docking results showed that15oformed key hydrogen bonds with Asn433 and Asp381. The picture of superposed conformation of 15o and homolog of ABBV-075 exhibited the combination difference between them, explaining the reason of activity gap, which would provide excellent ideas for further study.
Key words: BRD4 inhibitor; ABBV-075; synthesis and optimization; bioactivity; molecular docking
Chenhao Xu , Yunpeng Gong , Yaxin Chen , Qimeng Song , Jiao Li , Yichao Zheng , Wen Li , Kai Sun , Hongmin Liu . Design, Synthesis and Activity Evaluation of Novel Bromodomain-Containing Protein 4 (BRD4) Small Molecule Inhibitor Based on ABBV-075[J]. Chinese Journal of Organic Chemistry, 2021 , 41(4) : 1712 -1721 . DOI: 10.6023/cjoc202007059
| [1] | Smith, S.G.; Zhou, M.-M. ACS Chem. Biol. 2016, 11,598. |
| [2] | Filippakopoulos, P.; Picaud, S.; Mangos, M.; Keates, T.; Lambert, J.-P.; Barsyte-Lovejoy, D.; Felletar, I.; Volkmer, R.; Müller, S.; Pawson, T.; Gingras, A.-C.; Arrowsmith, C.H.; Knapp, S. Cell 2012, 149,214. |
| [3] | Lee, J.-E.; Park, Y.-K.; Park, S.; Jang, Y.; Waring, N.; Dey, A.; Ozato, K.; Lai, B.; Peng, W.; Ge, K. Nat. Commun. 2017, 8,2217. |
| [4] | Muller, S.; Filippakopoulos, P.; Knapp, S. Expert. Rev. Mol.Med. 2011, 13,e29. |
| [5] | Qin, Z.-Y.; Wang, T.; Su, S.; Shen, L.-T.; Zhu, G.-X.; Liu, Q.; Zhang, L.; Liu, K.-W.; Zhang, Y.; Zhou, Z.-H.; Zhang, X.-N.; Wen, L.-Z.; Yao, Y.-L.; Sun, W.-J.; Guo, Y.; Liu, K.-J.; Liu, L.; Wang, X.-W.; Wei, Y.-L.; Wang, J.; Xiao, H.-L.; Liu, P.; Bian, X.-W.; Chen, D.-F.; Wang, B. Cancer Res. 2019, 79,4869. |
| [6] | Wang, Q.; Sun, Y.; Li, T.; Liu, L.; Zhao, Y.; Li, L.; Zhang, L.; Meng, Y. Mol. Med. Rep. 2019, 19,499. |
| [7] | Ghosh, S.; Lora, J.M. Drug Discovery Today: Technol. 2016, 19,39. |
| [8] | Hargreaves, D.C.; Horng, T.; Medzhitov, R. Cell 2009, 138,129. |
| [9] | Liu, Z.; Wang, P.; Chen, H.; Wold, E.A.; Tian, B.; Brasier, A.R.; Zhou, J. J. Med. Chem. 2017, 60,4533. |
| [10] | Duan, Y.; Guan, Y.; Qin, W.; Zhai, X.; Yu, B.I. N.; Liu, H. MedChemComm 2019,10. |
| [11] | Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W.B.; Fedorov, O.; Morse, E.M.; Keates, T.; Hickman, T.T.; Felletar, I.; Philpott, M.; Munro, S.; McKeown, M.R.; Wang, Y.; Christie, A.L.; West, N.; Cameron, M.J.; Schwartz, B.; Heightman, T.D.; La Thangue, N.; French, C.A.; Wiest, O.; Kung, A.L.; Knapp, S.; Bradner, J.E. Nature 2010, 468,1067. |
| [12] | Dawson, M.A.; Prinjha, R.K.; Dittmann, A.; Giotopoulos, G.; Bantscheff, M.; Chan, W.-I.; Robson, S.C.; Chung, C.-w.; Hopf, C.; Savitski, M.M.; Huthmacher, C.; Gudgin, E.; Lugo, D.; Beinke, S.; Chapman, T.D.; Roberts, E.J.; Soden, P.E.; Auger, K.R.; Mirguet, O.; Doehner, K.; Delwel, R.; Burnett, A.K.; Jeffrey, P.; Drewes, G.; Lee, K.; Huntly, B.J. P.; Kouzarides, T. Nature 2011, 478,529. |
| [13] | Knapp, S.; Arruda, P.; Blagg, J.; Burley, S.; Drewry, D.H.; Edwards, A.; Fabbro, D.; Gillespie, P.; Gray, N.S.; Kuster, B.; Lackey, K.E.; Mazzafera, P.; Tomkinson, N.C. O.; Willson, T.M.; Workman, P.; Zuercher, W.J. Nat. Chem. Biol. 2013, 9,3. |
| [14] | Ember, S.W. J.; Zhu, J.-Y.; Olesen, S.H.; Martin, M.P.; Becker, A.; Berndt, N.; Georg, G.I.; Sch?nbrunn, E. ACS Chem. Biol. 2014, 9,1160. |
| [15] | Liu, S.; Yosief, H.O.; Dai, L.; Huang, H.; Dhawan, G.; Zhang, X.; Muthengi, A.M.; Roberts, J.; Buckley, D.L.; Perry, J.A.; Wu, L.; Bradner, J.E.; Qi, J.; Zhang, W. J. Med. Chem. 2018, 61,7785. |
| [16] | McDaniel, K.F.; Wang, L.; Soltwedel, T.; Fidanze, S.D.; Hasvold, L.A.; Liu, D.; Mantei, R.A.; Pratt, J.K.; Sheppard, G.S.; Bui, M.H.; Faivre, E.J.; Huang, X.; Li, L.; Lin, X.; Wang, R.; Warder, S.E.; Wilcox, D.; Albert, D.H.; Magoc, T.J.; Rajaraman, G.; Park, C.H.; Hutchins, C.W.; Shen, J.J.; Edalji, R.P.; Sun, C.C.; Martin, R.; Gao, W.; Wong, S.; Fang, G.; Elmore, S.W.; Shen, Y.; Kati, W.M. J. Med. Chem. 2017, 60,8369. |
| [17] | Faivre, E.J.; Wilcox, D.; Lin, X.; Hessler, P.; Torrent, M.; He, W.; Uziel, T.; Albert, D.H.; McDaniel, K.; Kati, W.; Shen, Y. Mol. Cancer Res. 2017, 15,35. |
| [18] | Li, Z.; Xiao, S.; Yang, Y.; Chen, C.; Lu, T.; Chen, Z.; Jiang, H.; Chen, S.; Luo, C.; Zhou, B. J. Med. Chem. 2020, 63,3956. |
| [19] | Sheppard, G.S.; Wang, L.; Fidanze, S.D.; Hasvold, L.A.; Liu, D.; Pratt, J.K.; Park, C.H.; Longenecker, K.; Qiu, W.; Torrent, M.; Kovar, P.J.; Bui, M.; Faivre, E.; Huang, X.; Lin, X.; Wilcox, D.; Zhang, L.; Shen, Y.; Albert, D.H.; Magoc, T.J.; Rajaraman, G.; Kati, W.M.; McDaniel, K.F. J. Med. Chem. 2020, 63,5585. |
| [20] | Brown, N. Mol. Inf. 2014, 33,458. |
| [21] | Li, Z.-H.; Yang, D.-X.; Geng, P.-F.; Zhang, J.; Wei, H.-M.; Hu, B.; Guo, Q.; Zhang, X.-H.; Guo, W.-G.; Zhao, B.; Yu, B.; Ma, L.-Y.; Liu, H.-M. Eur. J. Med. Chem. 2016, 124,967. |
| [22] | Xie, H.; Zeng, L.; Zeng, S.; Lu, X.; Zhang, G.; Zhao, X.; Cheng, N.; Tu, Z.; Li, Z.; Xu, H.; Yang, L.; Zhang, X.; Huang, M.; Zhao, J.; Hu, W. Eur. J. Med. Chem. 2012, 52,205. |
| [23] | Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95,2457. |
| [24] | Ramgren, S.D.; Hie, L.; Ye, Y.; Garg, N.K. Org. Lett. 2013, 15,3950. |
| [25] | Hu, Y.-H. WO 2018086585, 2018. |
| [26] | Hu, Y.-H. WO 2018086604, 2018. |
/
| 〈 |
|
〉 |