

Chinese Journal of Organic Chemistry >
Palladium-Catalyzed C8 Alkylation of 1-Naphthylamides and Its Application to the Synthesis of the Core Sturctures of Aporphine and Aristolactam Alkaloids
Received date: 2020-10-16
Revised date: 2020-11-11
Online published: 2020-12-05
Supported by
Zhejiang Provincial Natural Science Foundation of China(LY18B020010); Opening Project of the Zhejiang Provincial Top Key Discipline of Pharmaceutical Sciences(201705); Foundation of Wenzhou Medical University Renji College(RJRC14001)
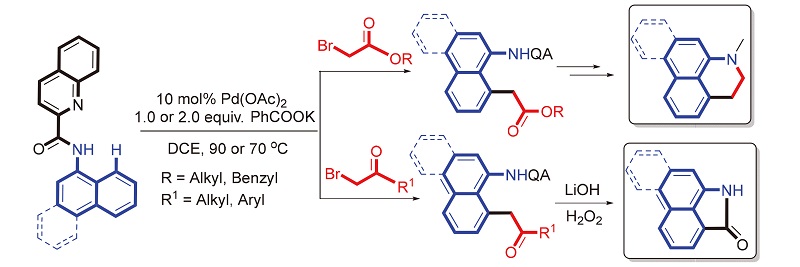
A practical methodology for the palladium-catalyzed regioselective alkylation of 8-C—H bonds in 1-naphthyl- amides containing a quinolinamide moiety as bidentate directing group with functionalized alkyl halides is reported. Various functionalized alkyl halides includingα-bromo esters and ketones can be employed as coupling partners, providing exclusively 8-alkyl-1-naphthylamine derivatives. In particular, the alkylated products with these ester and carbonyl groups can readily be further converted into the core structures of aporphine and aristolactam alkaloids respectively.
Honglei Jin , Fengxuan Jiang , Kai Cheng , Lehao Huang . Palladium-Catalyzed C8 Alkylation of 1-Naphthylamides and Its Application to the Synthesis of the Core Sturctures of Aporphine and Aristolactam Alkaloids[J]. Chinese Journal of Organic Chemistry, 2021 , 41(4) : 1691 -1702 . DOI: 10.6023/cjoc202010023
| [1] | For representative reviews on C—H functionalization, see: (a) Alberico, D.; Scott, M. E.; Lautens, M.; Chem. Rev. 2007, 107,174. |
| [1] | (b) Ackermann, L. Chem. Commun. 2010, 46,4866. |
| [1] | (c) Li, H.; Li, B.-J.; Shi, Z.-J. Catal. Sci. Technol. 2011, 1,191. |
| [1] | (d) Rouquet, G.; Chatani, N. Angew. Chem. Int. Ed. 2013, 52,11726. |
| [1] | (e) Daugulis, O.; Roane, J.; Tran, L.D. Acc. Chem. Res. 2015, 48,1053. |
| [1] | (f) Chen, Z.; Wang, B.; Zhang, J.; Yu, W.; Liu, Z.; Zhang, Y. Org. Chem. Front. 2015, 2,1107. |
| [1] | (g) He, G.; Wang, B.; Nack, W.A.; Chen, G. Acc. Chem. Res. 2016, 49,635. |
| [1] | (h) Dong, Z.; Ren, Z.; Thompson, S.J.; Xu, Y.; Dong, G. Chem. Rev. 2017, 117,9333. |
| [1] | (i) Saint-Denis, T.G.; Zhu, R.-Y.; Chen, G.; Wu, Q.-F.; Yu, J.-Q. Science 2018, 359,eaao4798. |
| [1] | (j) Sambiagio, C.; Sch?nbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; Maes, B.U. W.; Schnürch, M. Chem. Soc. Rev. 2018, 47,6603. |
| [1] | (k) Rej, S.; Ano, Y.; Chatani, N. Chem. Rev. 2020, 120,1788. |
| [1] | (l) Liao, G.; Wu, Y.-J.; Shi, B.-F. Acta Chim. Sinica 2020, 78,289. (in Chinese) |
| [1] | ( 廖港, 吴勇杰, 史炳锋, 化学学报, 2020, 78,289.) |
| [1] | (m) Liu, Y.-H.; Xia, Y.-N.; Shi, B.-F. Chin. J. Chem. 2020, 38,635. |
| [2] | For representative reviews on C—H functionalization logic in natural products and medicinal compounds, see: (a) Godula, K.; Sames, D.; Science 2006, 312,67. |
| [2] | (b) Gutekunst, W.R.; Baran, P.S. Chem. Soc. Rev. 2011, 40,1976. |
| [2] | (c) Yamaguchi, J.; Yamaguchi, A.D.; Itami, K. Angew. Chem. Int. Ed. 2012, 51,8960. |
| [2] | (d) Chen, D.Y. K.; Youn, S.W. Chem.-Eur. J. 2012, 18,9452. |
| [2] | (e) Cernak, T.; Dykstra, K.D.; Tyagarajan, S.; Vachal, P.; Krska, S.W. Chem. Soc. Rev. 2016, 45,546. |
| [2] | (f) Wang, W.; Lorion, M.M.; Shah, J.; Kapdi, A.R.; Ackermann, L. Angew. Chem. Int. Ed. 2018, 57,14700. |
| [3] | (a) Huang, L.; Li, Q.; Wang, C.; Qi, C. J. Org. Chem. 2013, 78,3030. |
| [3] | (b) Huang, L.; Sun, X.; Li, Q.; Qi, C. J. Org. Chem. 2014, 79,6720. |
| [4] | (a) Nadres, E.T.; Santos, G.I. F.; Shabashov, D.; Daugulis, O. J. Org. Chem. 2013, 78,9689. |
| [4] | (b) Yu, X.; Yang, F.; Wu, Y.; Wu, Y. Org. Lett. 2019, 21,1726. |
| [4] | (c) Shi, Y.; Yang, F.; Wu, Y. Org. Biomol. Chem. 2020, 18,4628. |
| [4] | (d) Li, Z.; Sun, S.; Qiao, H.; Yang, F.; Zhu, Y.; Kang, J.; Wu, Y.; Wu, Y. Org. Lett. 2016, 18,4594. |
| [4] | (e) Guan, D.; Han, L.; Wang, L.; Song, H.; Chu, W.; Sun, Z. Chem. Lett. 2015, 44,743. |
| [4] | (f) Wang, L.; Yang, M.; Liu, X.; Song, H.; Han, L.; Chu, W.; Sun, Z. Appl. Organomet. Chem. 2016, 30,680. |
| [4] | (g) Iwasaki, M.; Kaneshika, W.; Tsuchiya, Y.; Nakajima, K.; Nishihara, Y. J. Org. Chem. 2014, 79,11330. |
| [5] | For representative reviews on the biological activities of aporphine alkaloids,see: (a) Guinaudeau, H.; Leboeuf, M.; Cavé, A. J. Nat. Prod. 1994, 57,1033. |
| [5] | (b) Ríos, J.L.; Má?ez, S.; Giner, R.M.; Recio, M.C. In The Alkaloids: Chemistry and Biology,Ed.: Cordell, G. A., Academic Press, 1999, Vol.53, p.57. |
| [5] | For representative reviews on the biological activities of aristolactam alkaloids,see: (c) Kumar, V.; Poonam; Prasad, A.K.; Parmar, V.S. Nat. Prod. Rep. 2003, 20,565. |
| [5] | (d) Bentley, K.W. Nat. Prod. Rep. 2006, 23,444. |
| [6] | For representative examples on the biological activities of aporphine alkaloids,see: (a) Zhang, A.; Zhang, Y.; Branfman, A. R.; Baldessarini, R. J.; Neumeyer, J. L. J. Med. Chem. 2007, 50,171. |
| [6] | (b) Stevigny, C.; Bailly, C.; Quetin-Leclercq, J. Anti-Cancer Agents Med. Chem. 2005, 5,173. |
| [6] | (c) Mohamed, S.M.; Hassan, E.M.; Ibrahim, N.A. Nat. Prod. Res. 2009, 24,1395. |
| [6] | (d) Boustie, J.; Stigliani, J.-L.; Montanha, J.; Amoros, M.; Payard, M.; Girre, L. J. Nat. Prod. 1998, 61,480. |
| [7] | For representative examples on the biological activities of aristolactam alkaloids, see: (a) Chia, Y.-C.; Chang, F.-R.; Teng, C.-M.; Wu, Y.-C. J. Nat. Prod. 2000, 63,1160. |
| [7] | (b) Zhang, Y.-N.; Zhong, X.-G.; Zheng, Z.-P.; Hu, X.-D.; Zuo, J.-P.; Hu, L.-H. Bioorg. Med. Chem. 2007, 15,988. |
| [7] | (c) Lee, H.S.; Han, D.S. J. Nat. Prod. 1992, 55,1165. |
| [8] | Song, J.; Chen, W.; Zhao, Y.; Li, C.; Liang, G.; Huang, L. RSC Adv. 2016, 6,54984. |
| [9] | (a) De Kimpe, N.; Verhé, R. α-Haloketones, α-Haloaldehydes and α-Haloimines, Wiley, New York, 1988, p. 1. |
| [9] | (b) Yasuda, M.; Tsuji, S.; Shigeyoshi, Y.; Baba, A. J. Am. Chem. Soc. 2002, 124,7440. |
| [9] | (c) Malosh, C.F.; Ready, J.M. J. Am. Chem. Soc. 2004, 126,10240. |
| [9] | (d) Liu, C.; He, C.; Shi, W.; Chen, M.; Lei, A. Org. Lett. 2007, 9,5601. |
| [9] | (e) Lundin, P.M.; Fu, G.C. J. Am. Chem. Soc. 2010, 132,11027. |
| [9] | (f) Huang, K.; Li, G.; Huang, W.-P.; Yu, D.-G.; Shi, Z.-J. Chem. Commun. 2011, 47,7224. |
| [9] | (g) Mao, J.; Liu, F.; Wang, M.; Wu, L.; Zheng, B.; Liu, S.; Zhong, J.; Bian, Q.; Walsh, P.J. J. Am. Chem. Soc. 2014, 136,17662. |
| [9] | (h) Shu, W.-M.; Ma, J.-R.; Zheng, K.-L.; Wu, A.-X. Org. Lett. 2016, 18,196. |
| [9] | (i) Liu, W.; Cao, W.; Hu, H.; Lin, L.; Feng, X. Chem. Commun. 2018, 54,8901. |
| [10] | For representative examples on the α-halo esters and ketones can be employed as the alkylation reagent in C—H functionalization, see: (a) Hennessy, E. J.; Buchwald, S. L. J. Am. Chem. Soc. 2003, 125,12084. |
| [10] | (b) Liu, C.; Liu, D.; Zhang, W.; Zhou, L.; Lei, A. Org. Lett. 2013, 15,6166. |
| [10] | (c) Nakatani, A.; Hirano, K.; Satoh, T.; Miura, M. Chem.-Eur. J. 2013, 19,7691. |
| [10] | (d) Yu, D.-G.; de Azambuja, F.; Glorius, F. Angew. Chem. Int. Ed. 2014, 53,2754. |
| [10] | (e) Xie, C.; Dai, Z.; Niu, Y.; Ma, C. J. Org. Chem. 2018, 83,2317. |
| [10] | (f) Li, J.; Zhang, Z.; Tang, M.; Zhang, X.; Jin, J. Org. Lett. 2016, 18,3898. |
| [10] | (g) Zhou, J.; Li, J.; Li, Y.; Wu, C.; He, G.; Yang, Q.; Zhou, Y.; Liu, H. Org. Lett. 2018, 20,7645. |
| [11] | During preparation of the manuscript, similar reaction was reported by the Wu group: Wang, X.; Feng, C.; Yang, F.; Wu, Y. Org. Biomol. Chem. 2019, 17,4865. |
| [12] | He, G.; Lu, C.; Zhao, Y.; Nack, W.A.; Chen, G. Org. Lett. 2012, 14,2944. |
| [13] | Hase, T. Synthesis 1980,36. |
| [14] | (a) Zaitsev, V.G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127,13154. |
| [14] | (b) Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2010, 132,3965. |
| [14] | (c) Nadres, E.T.; Daugulis, O. J. Am. Chem. Soc. 2011, 134,7. |
| [14] | (d) Tran, L.D.; Daugulis, O. Angew. Chem., nt. Ed. 2012, 51,5188. |
| [14] | (e) He, G.; Chen, G. Angew. Chem., nt. Ed. 2011, 50,5192. |
| [15] | (a) Xie, Y.; Yang, Y.; Huang, L.; Zhang, X.; Zhang, Y. Org. Lett. 2012, 14,1238. |
| [15] | (b) Zhang, M.; Li, R.; Yang, Z.; Feng, R. Chin. J. Org. Chem. 2020, 40,714. (in Chinese) |
| [15] | ( 张梦帆, 李瑞鹏, 杨震, 冯若昆, 有机化学, 2020, 40,714.) |
| [16] | (a) Xie, A.; Cao, M.; Liu, Y.; Feng, L.; Hu, X.; Dong, W. Eur. J. Org. Chem. 2014,436. |
| [16] | (b) Li, Q.; Zhang, S.-Y.; He, G.; Ai, Z.; Nack, W.A.; Chen, G. Org. Lett. 2014, 16,1764. |
| [17] | DeRuiter, J.; Swearingen, B.E.; Wandrekar, V.; Mayfield, C.A. J. Med. Chem. 1989, 32,1033. |
| [18] | Jia, X.; Huang, Q.; Li, J.; Li, S.; Yang, Q. Synlett 2007,0806. |
| [19] | Yang, N.C.; Lenz, G.R.; Shani, A. Tetrahedron Lett. 1966, 7,2941. |
| [20] | Ying, J.; Fu, L.-Y.; Zhong, G.; Wu, X.-F. Org. Lett. 2019, 21,5694. |
/
| 〈 |
|
〉 |