

Chinese Journal of Organic Chemistry >
Facile Preparation of Aryl C-Glycosides by Nickel-Catalyzed Reductive Coupling of Glycosyl Halides with Aryl Halides
Received date: 2020-10-20
Revised date: 2020-11-12
Online published: 2020-12-05
Supported by
National Natural Science Foundation of China(21871173)
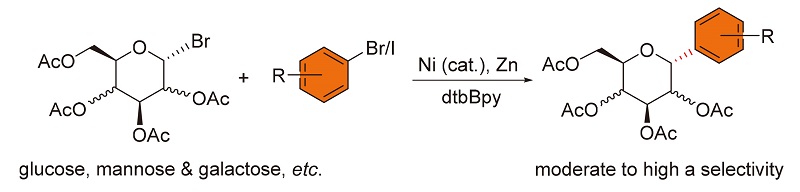
The preparation of C-aryl glycosides via mild Ni/bipyridine-catalyzed reductive arylation of C(1)-glycosyl halides with electron-deficient aryl bromides was developed. Moderate to high α-selectivities were achieved for C-glycosides. A broad range of aryl halides including electron-rich aryl iodides were employed to yield C-aryl glycosides in 40%~5% yields. This method can be scaled up on a gram scale by lowering the loading of nickel catalyst to 2 mol%.
Yuren Sun , Jiandong Liu , Quan Lin , Ken Yao , Weiqi Tong , Qun Qian . Facile Preparation of Aryl C-Glycosides by Nickel-Catalyzed Reductive Coupling of Glycosyl Halides with Aryl Halides[J]. Chinese Journal of Organic Chemistry, 2021 , 41(4) : 1551 -1562 . DOI: 10.6023/cjoc202010027
| [1] | For a recent review, see: (a) Yang, Y., Yu, B., Chem. Rev. 2017, 117,12281. |
| [1] | (b) Ji, Y.; Yao, H.; Liu, Y.; Huang, N.; Liu, M. Chin. J. Org. Chem. 2020, 40,2051. (in Chinese) |
| [1] | ( 吉玉, 姚辉, 柳乂, 黄年玉, 刘明国, 有机化学, 2020, 40,2051.) |
| [2] | (a) Kulkarni, S.S.; Gervay-Hague, J. Org. Lett. 2006, 8,5765. |
| [2] | (b) Synthetic Strategies toward SGLT2 Inhibitors: Aguill?n, A.R.; Mascarello, A.; Segretti, N. D.; de Azevedo, H. F. Z.; Guimaraes, C. R. W.; Miranda, L. S. M.; de Souza, R. O. M. A. Org. Process Res. Dev. 2018, 22,467. |
| [3] | For Ni-catalyzed cross-coupling of glycosyl halides, see: (a) Gong, H.; Sinisi, R.; Gagné, M. R. J. Am. Chem. Soc. 2007, 129,1908. |
| [3] | (b) Gong, H.; Gagné, M.R. J. Am. Chem. Soc. 2008, 130,12177. |
| [4] | Lemaire, S.; Houpis, I.N.; Xiao, T.; Li, J.; Digard, E.; Gozlan, C.; Liu, R.; Gavryushin, A.; Coura Diène, C.; Wang, Y.; Farina, V.; Knochel, P. Org. Lett. 2012, 14,1480. |
| [5] | Laksmikanta, Adak, L.; Kawamura, S.; Toma, G.; Takenaka, T.; Isozaki, K.; Takaya, H.; Orita, A.; Li, H.C.; Shing, T.K. M.; Nakamura, M. J. Am. Chem. Soc. 2017, 139,10693. |
| [6] | Nicolas, L.; Angibaud, P.; Stansfield, I.; Bonnet, P.; Meerpoel, L.; Reymond, S.; Cossy, J. Angew. Chem., Int. Ed. 2012, 51,11101. |
| [7] | (a) Zhu, F.; Rodriguez, J.; Yang, T.; Kevlishvili, I.; Miller, E.; Yi, D.; O'Neill, S.; Rourke, M.J.; Liu, P.; Walcza, M.A. J. Am. Chem. Soc. 2017, 139,17908. |
| [7] | (b) Yi, D.; Zhu, F.; Walczak, M.A. Org. Lett. 2018, 20,1936. |
| [8] | For additional samples, see: (a) Li, Y.; Fan, Y.; Jia, Q. Chin. J. Org. Chem. 2019, 39,350. (in Chinese) |
| [8] | ( 李娅琼, 范玉航, 贾乾发, 有机化学, 2019, 39,350.) |
| [8] | (b) Wang, Q.; Zhu, W.; Sun, Q.; He, G.; Chen, G. Chin. J. Chem. 2021, 39,571. |
| [8] | Wang, H.; Wu, P.; Zhao, X.; Zeng, J.; Wan, Q. Acta Chim. Sinica 2019, 77,231. (in Chinese) |
| [8] | ( 王浩, 吴品儒, 赵祥, 曾静, 万谦, 化学学报, 2019, 77,231.) |
| [9] | Liu, J.; Gong, H. Org. Lett. 2018, 20,7991. |
| [10] | Liu, J.; Lei, C.; Gong, H. Sci. China: Chem. 2019, 62, 1492. |
| [11] | Low to moderate α-selectivities were also observed for the coupling of glucosyl bromide with acyl electrophiles using bipy ligands, see: (a) Zhao, C.; Jia, X.; Wang, X.; Xue, W. J. Am. Chem. Soc. 2014, 136,17645. |
| [11] | (b) Jia, X.; Zhang, X.; Qian, Q.; Gong, H. Chem. Commun. 2015, 51,10302. |
| [11] | (c) Zheng, M.; Xue, W.; Xue, T.; Gong, H. Org. Lett. 2016, 18,6152. |
| [12] | Very recently, use of Bipy ligands for Ni-catalyzed photo-redox C-saccharide formation was reported without glucosyl substrates: (a) Very, recently, Badir, S. O.; Dumoulin, A.; Matsui, J. K.; Molander, G. A. Angew. Chem., Int. Ed. 2018, 57,6610. |
| [12] | (b) Dumoulin, A.; Matsui, J.K.; lvaro Gutiérrez-Bonet, á.; Molander, G.A. Angew. Chem., Int. Ed. 2018, 57,6614. |
| [13] | Jones, G.D.; Martin, J.L.; McFarland, C.; Allen, O.R.; Hall, R.E.; Haley, A.D.; Brandon, R.J.; Konovalova, T.; Desrochers, P.J.; Pulay, P.; Vicic, D.A. J. Am. Chem. Soc. 2006, 128,13175. |
| [14] | Wang, X.; Zhang, L.; Byrne, D.; Senanayake, C.H. Org. Lett. 2014, 16,4090. |
| [15] | Suh, S.; Chen, S.; Mandal, M.; Stahl, S.S. J. Am. Chem. Soc. 2020, 142,11388. |
| [16] | Liu, J.; Gong, H.; Lei, C.; Qian, Q. CN 108530409, 2018. |
| [17] | Meng, W.; Ellsworth, B.A.; Nirschl, A.A.; Biller, S.A. J. Med. Chem. 2008, 51,1145. |
| [18] | Sadurni, A.; Kehr, G.; Ahlqvist, M.; Gilmour, R. Chem.-Eur. J. 2018, 24,2832. |
/
| 〈 |
|
〉 |