

Chinese Journal of Organic Chemistry >
Novel 3-Thioether-4-indolimino-4H-1,2,4-triazole Derivatives Bearing Pyridyl Moiety: Design, Synthesis and Bioactivity Evaluation in vitro
Received date: 2020-08-31
Revised date: 2020-11-06
Online published: 2020-12-10
Supported by
Natural Science Foundation of China(21867004); State Key Laboratory of Functions and Applications of Medicinal Plants(FAMP201801K); Guizhou Science technology program for Platform and Talents(No.20185781)()
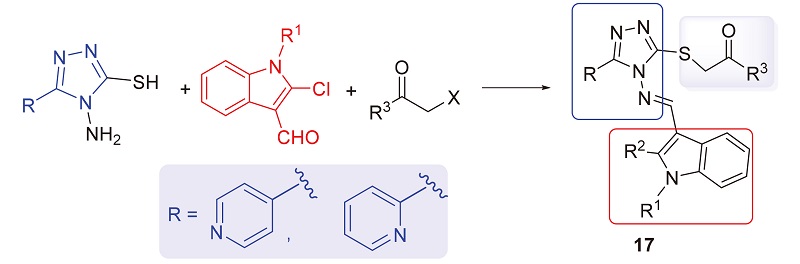
A series of novel 3-thioether-4-indolimino-4H-1,2,4-triazole derivatives bearing pyridyl moiety (17a~17r) have been designed, synthesized and evaluated for antiproliferative activities against four human cancer cells A549, PC-3, HepG2 and K562, and normal rat kidney cells NRK-52E using methyl thiazolyl tetrazolium (MTT) assay. The results showed that some compounds exhibited moderate antiproliferative activities against four cancer cells. Among these derivatives, ethyl (E)-2-((4-(((2-chloro-1-ethyl-1H-indol-3-yl)methylene)amino)-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetate (17k) showed the most potent antiproliferative activity against PC-3 cells with IC50value of 9.90 μmol/L, and the toxicities of compound 17k to NRK-52E cells were significantly lower than the positive control 5-fluorouracil. Meanwhile, the cell migration assay, 4,6-diamidino-2-phenylindole (DAPI) staining, mitochondrial membrane potential analysis, cell apoptosis and cell cycle analysis were carried out to further studied the mechanism of compound 17k, which demonstrated that compound 17k can effectively inhibit tumor cell migration and significantly induce cell apoptosis, arrest PC-3 cells in the G2 stage in a dose-dependent manner. In addition, the antibacterial tests of target compounds against Xanthomonas oryzae pv. oryzae (Xoo) were also explored. The preliminary antibacterial activity results showed that compounds 17b, 17e and 17h displayed a noteworthy inhibition rate against Xoo compared to standard drugs bismerthiazol (BMT) and thiodiazole copper (TDC) at 50 and 100 μg/mL.
Key words: 1,2,4-triazole; indole; antiproliferative activity; bactericidal activity
Yayun Qi , Jiamin Liu , Chenpeng Li , Weinan Hu , Siyu Tang , Lihui Shao , Zhenchao Wang , Guiping Ouyang . Novel 3-Thioether-4-indolimino-4H-1,2,4-triazole Derivatives Bearing Pyridyl Moiety: Design, Synthesis and Bioactivity Evaluation in vitro[J]. Chinese Journal of Organic Chemistry, 2021 , 41(4) : 1670 -1682 . DOI: 10.6023/cjoc202008057
| [1] | Chabner, B.A.; Roberts, T.G. Nat. Rev. Cancer 2005, 5,65. |
| [2] | Aytac, P.S.; Durmaz, I.; Houston, D.R.; Cetin-Atalay, R.; Tozkoparan, B. Bioorg. Med. Chem. 2016, 24,858. |
| [3] | Kulabas, N.; Tatar, E.; Ozakp?nar, O.B.; Ozsavci, D.; Pannecouque, C.; Clercq, E.D.; Kü?ükgüzel, I. Eur. J. Med. Chem. 2016, 121,58. |
| [4] | El-Sherief, H.A. M.; Youssif, B.G. M.; Abbas Bukhari, S.N.; Abdelazeem, A.H.; Abdel-Aziz, M.; Abdel-Rahman, H.M. Eur. J. Med. Chem. 2018, 156,774. |
| [5] | Boraei, A.T. A.; Singh, P.K.; Sechi, M.; Satta, S. Eur. J. Med. Chem. 2019, 182,111621. |
| [6] | Aboeldahab, A.M. A.; Beshr, E.A. M.; Shoman, M.E.; Aly, O.M. Eur. J. Med. Chem. 2018, 146,79. |
| [7] | Khan, I.; Zaib, S.; Ibrar, A.; Ahmad, S.; Furtmann, N.; Hameed, S.; Simpson, J.; Bajorath, J.; Iqbal, J. Eur. J. Med. Chem. 2014, 78,167. |
| [8] | Kamel, M.M.; Abdo, N.Y. M. Eur. J. Med. Chem. 2014, 86,75. |
| [9] | Sekhar, M.M.; Nagarjuna, U.; PadmavathiJ, V.; Padmaja, A.; Vasudeva Reddy, N.; Vijaya, T. Eur. J. Med. Chem, 2018, 145,1. |
| [10] | Fan, Y.Ke.; L., X.; Li, M. J. Heterocycl. Chem. 2018, 55,791. |
| [11] | Almajan, G.L.; Barbuceanu, S.F.; Saramet, I.; Draghici, C.; Eur. J. Med. Chem. 2010, 45,3191. |
| [12] | Aouad, M.R.; Mayaba, M.M.; Naqvi, A.; Bardaweel, S.K.; Al-blewi, F.F.; Messali, M.; Rezki, N. Chem. Cent. J. 2017, 11,117. |
| [13] | Prakash, O.; Aneja, D.K.; Hussai, K.; Lohan, P.; Ranjan, P.; Arora, S.; Sharmac, C.; Aneja, K,P. Eur. J. Med. Chem. 2011, 46,5065. |
| [14] | Sarigol, D.; Uzgoren-Baran, A.; Tel, B.C.; Somuncuoglu, E.I.; Kazkayas, I.; Ozadali-Sari, K.; Unsal-Tan, O.; Okay, G.; Ertan, M.; Tozkoparan, B. Bioorg. Med. Chem. 2015, 23,2518. |
| [15] | Sumangala, V.; Poojary, B.; Chidananda, N.; Arulmoli, T.; Shenoy, S. Med. Chem. Res. 2013, 22,2921. |
| [16] | Ahirwar, J.; Ahirwar, D.; Lanjhiyana, S.; Jha, A.K.; Dewangan, D.; Badwaik, H. J. Heterocycl. Chem. 2018, 55,2130. |
| [17] | Wang, B.L.; Shi, Y.X.; Ma, Y.; Liu, X.H.; Li, Y.H.; Song, H.B.; Li, Z.M. J. Agric. Food Chem. 2010, 58,5515. |
| [18] | Fan, C.C.; Jiao, S.L.; Qin, M.; Zou, Z.H. ChemistrySelect 2019, 4,8593. |
| [19] | Aly, A.A. A.; Hassan, A.; Makhlouf, M.M.; Br?se, S. Molecules 2020, 25.3036. |
| [20] | Cheng, Y.N.; Jiang, Z.H.; Sun, L.S. Eur. J. Med. Chem., 2020, 200,112463. |
| [21] | Demirbas, N.; Demirbas, A.; Karaoglu, S.A.; Celik, E. ARKIVOC 2005,75. |
| [22] | Tian, K.; Meng, J.; Gan, Y.Y.; Li, X.Q.; Wu, S.Q.; Chen, J.; Qi, Y.Y.; Hu, W.N.; Wang, Z.C.; OuYang, G.P. Chin. J. Org. Chem. 2018, 38,2657. (in Chinese) |
| [22] | ( 田坤, 孟娇, 甘宜远, 李小琴, 巫受群, 陈洁, 漆亚云, 胡伟男, 王贞超, 欧阳贵平, 有机化学, 2018, 38,2657.) |
| [23] | Li, W.; Qi, Y.Y.; Wang, Y.Y.; Gan, Y.Y.; Shao, L.H.; Zhang, L.Q.; Tang, Z.H.; Zhu, M.; Tang, S.Y.; Wang, Z.C.; OuYang, G.P. J. Heterocycl. Chem. 2020, 57,2548. |
| [24] | Li, X.Q.; Gan, Y.Y.; Meng, J.; Li, W.; Chen, J. Qi, Y.Y.; Tian, K.; OuYang, G.P.; Wang, Z.C. J. Heterocycl. Chem. 2018, 55,1382. |
| [25] | Tian, K.; Li, X.Q.; Zhang, L.; Gan, Y.Y.; Wu, S.Q.; Wan, J.L.; Xu, Y.; Cai, C.T.; OuYang, G.P.; Wang, Z.C. Chem. Pap. 2019, 73,17. |
| [26] | Hassan, A.Y.; Sarg, M.T.; El‐Sebaey, S.A. J. Heterocycl. Chem. 2020, 57,694. |
| [27] | Parameshwarappa, G.; Lingamani, J.; Patil, S.B.; Goudgaon, A.M. Heterocycl. Commun. 2009, 15,343. |
| [28] | Li, Q.; Wang, Y.; Hu, M.J.; Chen, P.; You, W.W.; Zhao, P. Chin. J. Org. Chem. 2017, 37,967. (in Chinese) |
| [28] | ( 黎秋, 汪雨, 陈鹏, 游文玮, 赵培亮, 有机化学, 2017, 37,967.) |
| [29] | Das Mukherjee, D.; Kumar, N.M.; Tantak, M.P.; Das, A.; Ganguli, A.; Datta, S.; Kumar, D.; Chakrabarti, G. Biochemistry 2016, 55,3020. |
| [30] | Wu, S.Q.; Li, X.Q.; Meng, J. Gan, Y.Y.; Tian, K.; Wang, Z.C.; OuYang, G.P. Chin. J. Org. Chem. 2018, 38,1447. (in Chinese) |
| [30] | ( 巫受群, 李小琴, 孟娇, 甘宜远, 田坤, 王贞超, 欧阳贵平, 有机化学, 2018, 38,1447.) |
| [31] | Zhang, B.; Li, Y.H.; Liu, Y.; Chen, Y.R.; Pan, E.S.; You, W.W.; Zhao, P.L. Eur. J. Med. Chem. 2015, 103,335. |
/
| 〈 |
|
〉 |