

Chinese Journal of Organic Chemistry >
Synthesis and Antifungal Activity of 3,7-Dimethyl-7-hydroxy-2-octen-6-olide Analogues
Received date: 2020-10-09
Revised date: 2020-11-20
Online published: 2020-12-19
Supported by
National Natural Science Foundation of China(21772229); National Natural Science Foundation of China(21172254)
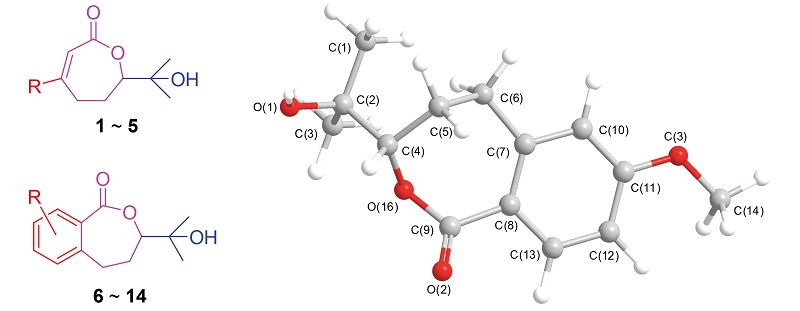
The synthesis of racemic and optical 3,7-dimethyl-7-hydroxy-2-octen-6-olide analogues has been achieved via epoxidation-lactonization procedure and Sharpless asymmetric dihydroxylation as the key steps in 55%~90% overall yields, respectively. Their structures were fully characterized by 1H NMR, 13C NMR, HRMS data, and X-ray diffraction analysis. Their antifungal activities were evaluated, and showed that the EC50values of the most active compounds 3-phenyl-7- methyl-7-hydroxy-2-octen-6-olide (4) and 3-(furan-2-yl)-7-methyl-7-hydroxy-2-octen-6-olide (5) were in the range of 0.5~20.0 μg/mL against the tested six phytopathgens, and they were the lead structures to be optimized.
Hongbo Dong , Weiwei Wang , Yu Zhao , Xinlei Liu , Ming'an Wang . Synthesis and Antifungal Activity of 3,7-Dimethyl-7-hydroxy-2-octen-6-olide Analogues[J]. Chinese Journal of Organic Chemistry, 2021 , 41(4) : 1646 -1657 . DOI: 10.6023/cjoc202010011
| [1] | Schulz, S.; Hotling, S. Nat. Prod. Rep. 2015, 32,1042. |
| [2] | Gabriela, L.; Gallardo, N.I. P.; Cabrera, G.M. Phytochem. Lett. 2008, 1,155. |
| [3] | Yang, Y.; Jiang, J.Z.; Qimei, L.B.; Yan, X.J.; Zhao, J.X.; Yuan, H.Z.; Qin, Z.H.; Wang, M.A. Molecules 2010, 15,7075. |
| [4] | Davies-Coleman, M.T.; Rivett, D.E. A. Prog. Chem. Org. Nat. Prod. 1989, 55,1. |
| [5] | Collett, L.A.; Davies-Coleman, M.T.; Rivett, D.E. A. Prog. Chem. Org. Nat. Prod. 1998, 75,182. |
| [6] | Pereda-Miranda, R.; Fragoso-Serrano, M.; Cerda-Garcia-Rojas, C.M. Tetrahedron 2001, 57,47. |
| [7] | Hoffmann, H.M. R.; Rabe, J. Angew. Chem. Int. Ed. 1985, 24,94. |
| [8] | Marco, J.A.; Carda, M.; Murga, J.; Falomir, E. Tetrahedron 2007, 63,2929. |
| [9] | Boucard, V.; Broustal, G.; Campagne, J.M. Eur. J. Org. Chem. 2007,225. |
| [10] | Gerard, R. Tetrahedron 1995, 51,2777. |
| [11] | Helena, M.C.; Fernanda, I.B.; Myrian, K.S.; Luiz, S.L. J. Quim. Nova 2008, 31,885. |
| [12] | Helena, M.C.; Fernanda, I.B.; Luiz, S.L. J. Synthesis 2007,3261. |
| [13] | Shiina, I. Chem. Rev. 2007, 107,239. |
| [14] | Chang, H.-T.; Jayanth, T.T.; Wang, C.-C.; Cheng, C.-H. J. Am. Chem. Soc. 2007, 129,12032. |
| [15] | Ebine, M.; Suga, Y.; Fuwa, H.; Sasaki, M. Org. Biomol. Chem. 2010, 8,39. |
| [16] | Emily, B.P.; Tendai, G.; Sonbinh, T.N. Org. Lett. 2008, 10,5613. |
| [17] | van, As, B.A. C.; Chan, D.; Kivit, P.J. J.; Palmans, A.R. A.; Meijer, E.W. Tetrahedron: Asymmetry 2007, 18,787. |
| [18] | Maxime, A.S.; Huub, K.; Anthony, L.S. Acta Crystallogr. 2008, E64,o607. |
| [19] | Fukuzawa, A.; Masamune, T. Tetrahedron Lett. 1981, 22,4081. |
| [20] | Cremer, D.J.; Pople, A. J. Am. Chem. Soc. 1975, 97,1354. |
| [21] | Wandjia, J.; Wansi, J.D.; Fuendjiep, V.; Dagne, E.; Mulholland, D.A.; Tillequin, F.; Fomum, Z.T.; Sondengam, B.L.; Nkeh, B.C.; Njamen, D. Phytochemistry 2000, 54,811. |
| [22] | Nkeh, B.C.; Njamen, D.; Wandjia, J.; Fomum, Z.T.; Dongmo, A.; Nguelefack, T.B.; Wansi, J.D.; Kamanyi, A. Pharm. Biol. 2003, 41,26. |
| [23] | Jhulki, S.; Seth, S.; Mondal, M.; Moorthy, J.N. Tetrahedron 2014, 70,2286. |
| [24] | Kadnikov, D.V.; Larock, R.C. Mendeleev Commun. 2007, 17,74. |
| [25] | Cheng, Y.A.; Chen, T.; Tan, C.K.; Heng, J.J.; Yeung, Y.Y. J. Am. Chem. Soc. 2012, 134,16492. |
| [26] | Wang, L.; Ren, Z.-L.; Ding, M.-W. J. Org. Chem. 2015, 80,641. |
| [27] | Arcadi, A.; Fabrizi, G.; Goggiamani, A.; Marinelli, F. J. Org. Chem. 2015, 80,6986. |
| [28] | Matsuda, T.; Shigeno, M.; Murakami, M. Org. Lett. 2008, 10,5219. |
| [29] | Zarrabi, S.; Mahmoodi, N.O.; Tabatabaeian, K.; Zanjanchi, M.A. Chin. Chem. Lett. 2009, 20,1400. |
| [30] | Dong, H. B.; Yang, M. Y.; Jiang, J. Z.; Wang, M. A. J. Asian Nat. Prod. Res. 2013, 15,880. |
| [31] | Dong, H. B.; Yang, M. Y.; Tang, B.; Wang, M. A. Chin. J. Org. Chem. 2014, 34,2350. (in Chinese) |
| [31] | ( 董宏波, 杨明艳, 汤博, 王明安, 有机化学, 2014, 34,2350.) |
| [32] | Zhao, J.; Dong, H. B.; Yang, M. Y.; Du, J.; Jiang, J. Z.; Wang, M. A. J. Asian Nat. Prod. Res. 2014, 16,312. |
| [33] | Wang, W.W.; Zhao, Y.; Liu, X.L.; Geng, R.; Wang, M.A. Chin. J. Org. Chem., 2019, 39,1129. (in Chinese) |
| [33] | ( 王卫伟, 赵宇, 刘鑫磊, 耿瑞, 王明安, 有机化学, 2019, 39,1129.) |
| [34] | Srivastava, A.K.; Song, H.; Park, S.B. Synthesis 2011,1708. |
| [35] | Gesinski, M.R.; Tadpetch, K.; Rychnovsky, S.D. Org. Lett. 2009, 11,5342. |
| [36] | Matsuda, T.; Shigeno, M.; Murakami, M. Org. Lett. 2008, 10,5219. |
| [37] | Chang, H.T.; Jeganmohan, M.; Cheng, C.H. Chem. Commun. 2005,4955. |
| [38] | Roux, M.C.; Paugam, R.; Rousseau, G. J. Org. Chem. 2001, 66,4304. |
| [39] | Wong, O.A.; Shi, Y. Chem. Rev. 2008, 108,3958. |
| [40] | Dong, H.B.; Yang, M.Y.; Liu, B.; Wang, M.A. J. Asian Nat. Prod. Res. 2014, 16,847. |
| [41] | Kolb, H.C.; Van Nieuwenhze, M.S.; Sharpless, K.B. Chem. Rev. 1994, 94,2483. |
| [42] | Dong, H.B.; Yang, M.Y.; Zhang, X.T.; Wang, M.A. Tetrahedron: Asymmetry 2014, 25,610. |
| [43] | Jorge, G.-F.; Juan, M.; Miguel, C.; Alberto, M.J. Org. Lett. 2006, 8,2695. |
| [44] | Narender, T.; Sarkar, S.; Rajendar, K.; Tiwari, S. Org. Lett. 2011, 13,6140. |
| [45] | Inanaga, J.; Hirata, K.; Saeki, H.; Katsuki, T.; Yamaguchi, M. Bull. Chem. Soc. Jpn. 1979, 52,1989. |
| [46] | Yadav, J.S.; Rajendar, G.; Rao, R.S.; Pabbaraja, S. J. Org. Chem. 2013, 78,8524. |
| [47] | Ikuko, O.; Takenori, K.; Yoel, K.; Hiroshi, K. J. Am. Chem. Soc. 1991, 113,4092. |
| [48] | Tang, B.; Guan, A.Y.; Zhao, Y.; Jiang, J.Z.; Wang, M.A.; Zhou, L.G. Chin. J. Chem. 2017, 35,1133. |
| [49] | Zhao, Y.; Tang, B.; Guan, A.Y.; Wang, W.W.; Zhang, Z.H.; Wang, M.A. Synthesis 2017, 49,4663. |
| [50] | Geng, R.; Zhao, Y.; Li, Y.H.; Liu, X.L.; Wang, M.A. Chin. J. Org. Chem. 2019, 39,3574. (in Chinese) |
| [50] | ( 耿瑞, 赵宇, 李益豪, 刘鑫磊, 王明安, 有机化学, 2019, 39,3574.) |
/
| 〈 |
|
〉 |