

Chinese Journal of Organic Chemistry >
N-Heterocyclic Carbene (NHC)/Ag(I) Co-catalyzed Synthesis of 2-Oxo-2-arylethyl Aryl Formates
Received date: 2020-08-20
Revised date: 2020-10-13
Online published: 2020-12-24
Supported by
National Science Foundation of China(2177020721); National Science Foundation of China(21871113); Natural Science Foundation of the Jiangsu Higher Education Institution(17KJA150003)
An N-heterocyclic carbene (NHC)/Ag(I) co-catayzed efficient synthesis of 2-oxo-2-arylethyl aryl formates was realized by the reaction of aryl aldehydes with (bromoethynyl)benzenes. This method features broad substrate scope, ready availability of starting materials and operational simplicity, which gives an alternative access to α-acyloxycarbonyl derivatives.
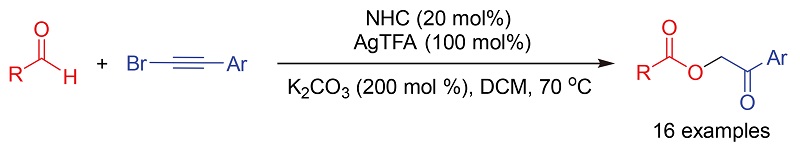
Li Cheng , Wenrong Wang , Yuqian Sun , Tuanjie Li , Chenxia Yu , Changsheng Yao . N-Heterocyclic Carbene (NHC)/Ag(I) Co-catalyzed Synthesis of 2-Oxo-2-arylethyl Aryl Formates[J]. Chinese Journal of Organic Chemistry, 2021 , 41(4) : 1607 -1613 . DOI: 10.6023/cjoc202008035
| [1] | Lin, L.; Mulholland, N.; Wu, Q.-Y.; Beattie, D.; Huang, S.-W.; Irwin, D.; Clough, J.; Gu, Y.-C.; Yang, G.-F. J. Agric. Food Chem. 2012, 60,4480. |
| [2] | Wang, X.; Sena Filho, J.G.; Hoover, A.R.; King, J.B.; Ellis, T.K.; Powell, D.R.; Cichewicz, R.H. J. Nat. Prod. 2010, 73,942. |
| [3] | Wang, H.; Wang, Y.; Wang, W.; Fu, P.; Liu, P.; Zhu, W. J. Nat. Prod. 2011, 74,2014. |
| [4] | (a) Sabbah, D.A.; Saada, M.; Khalaf, R.A.; Bardaweel, S.; Sweidan, K.; Al-Qirim, T.; Al-Zughier, A.; Halim, H.A.; Sheikh, G.A. Bioorg. Med. Chem. Lett. 2015, 25,3120. |
| [4] | (b) Che, Y.; Wen, D.; Huang, Z.; Huang, M.; Luo, Y.; Liu, B.; Lu, H.; Wu, Y.; Peng, Y.; Zhang, J. Bioorg. Med. Chem. Lett. 2012, 22,6867. |
| [5] | Dai, L.; Yu, S.; Xiong, W.; Chen, Z.; Xu, T.; Shao, Y.; Chen, J. Adv. Synth. Catal. 2020, 362,1893. |
| [6] | Kim, S.H.; Jang, M.; Moon, D.Y.; Park, B.S. Tetrahedron Lett. 2018, 59,4245. |
| [7] | Arai, M.A.; Kofuji, Y.; Tanaka, Y.; Yanase, N.; Yamaku, K.; Fuentes, R.G.; Karmakar, U.K.; Ishibashi, M. Org. Biomol. Chem. 2016, 14,3061. |
| [8] | (a) Funk, P.; Motyka, K.; D?ubák, P.; Znojek, P.; Gurská, S.; Kusz, J.; McMaster, C.; Hajdúch, M.; Soural, M. RSC Adv. 2015, 5,48861. |
| [8] | (b) Kadri?, J.; Motyka, K.; D?ubák, P.; Hajdúch, M.; Soural, M. Tetrahedron Lett. 2014, 55,3592. |
| [9] | (a) Prasad, P.K.; Reddi, R.N.; Arumugam, S. Org. Biomol. Chem. 2018, 16,9334. |
| [9] | (b) Zhou, X.; Ma, H.; Cao, J.; Liu, X.; Huang, G.; Org. Biomol. Chem. 2016, 14,10070. |
| [9] | (c) Zhu, Y.; Zheng, Y.; Song, W.; Wei, B.; Xuan, L. Tetrahedron Lett. 2018, 59,368. |
| [9] | (d) Zhu, M.; Wei, W.; Yang, D.; Cui, H.; Sun, X.; Wang, H. Org. Biomol. Chem. 2016, 14,10998. |
| [10] | (a) Kreibich, M.; Gemander, M.; Peter, D.; Yadav, D.; Koning, C.; Fernandes, M.; Green, I.; van Otterlo, W.; Brückner, R. Eur. J. Org. Chem. 2020, 19,2929. |
| [10] | (b) Liu, L.; Feng, S.; Li, C. ACS Sustainable Chem. Eng. 2016, 4,6754. |
| [10] | (c) Hu, Y.; Chen, J.; Le, Z.G.; Chen, Z.C.; Zheng, Q.G. Chin. Chem. Lett. 2005, 16,903. |
| [10] | (d) Wang, X.; Li, G.; Yang, Y.; Jiang, J.; Feng, Z.; Zhang, P. Chin. Chem. Lett. 2020, 31,711. |
| [11] | Mondal, B.; Sahoo, S.C.; Pan, S.C. Eur. J. Org. Chem. 2015, 14,3135. |
| [12] | Wang, J.-L.; Wang, J.-Q.; He, L.-N.; Dou, X.-Y.; Wu, F. Green Chem. 2008, 10,1218. |
| [13] | Ji, K.; Zhao, Y.; Zhang, L. Angew. Chem. Int. Ed. 2013, 52,6508. |
| [14] | Mu, Y.; Chen, Y.; Gao, Y.; Sun, J.; Iqbal, Z.; Wan, Y.; Yang, M.; Yang, Z.; Tang, D. ChemistrySelect 2020, 5,1705. |
| [15] | Tian, L.; Guo, Y.; Wei, L.; Wan, J.-P.; Sheng, S. Asian J. Org. Chem. 2019, 8,1484. |
| [16] | Li, J.; Yang, Z.; Yang, T.; Yi, J.; Zhou, C. New J. Chem. 2018, 42,1581. |
| [17] | Chen, C.; Liu, W.; Zhou, P.; Liu, H. RSC Adv. 2017, 7,20394. |
| [18] | Tan, L.; Chen, C.; Liu, W. Beilstein J. Org. Chem. 2017, 13,1079. |
| [19] | (a) Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Chem. Rev. 2015, 115,9307. |
| [19] | (b) Enders, D.; Niemeier, O.; Henseler, A. Chem. Rev. 2007, 107,5606. |
| [19] | (c) Wang, Z.; Li, R.; Qian, H.; Yao, C. Chin. J. Org. Chem. 2019, 39,2075. (in Chinese) |
| [19] | ( 王占林, 李如一, 钱辉旻, 姚昌盛, 有机化学, 2019, 39,2075.) |
| [19] | (d) Yao, C.; Xiao, Z.; Liu, R.; Li, T.; Jiao, W.; Yu, C. Chem. Eur. J. 2013, 19,456. |
| [19] | (e) Li, S.; Yang, W.; Luo, X.; Yao, C. Chin. J. Org. Chem. 2019, 39,1404. (in Chinese) |
| [19] | ( 李莎, 杨雯涵, 罗鲜, 姚昌盛, 有机化学, 2019, 39,1404.) |
| [19] | (f) Li, S.; Xu, J.; Luo, X.; Yang, W.; Yao, C. Chin. J. Org. Chem. 2020, 40,470. (in Chinese) |
| [19] | ( 李莎, 徐嘉煜, 罗鲜, 杨雯涵, 姚昌盛, 有机化学, 2020, 40,470.) |
| [19] | (g) Zhang, Y.; Xing, F.; Feng, Z.; Du, G.; Gu, C.; He, L. Chin. J. Org. Chem. 2020, 40,1608. (in Chinese) |
| [19] | ( 张阳, 邢芬, 冯泽男, 杜广芬, 顾承志, 何林, 有机化学, 2020, 40,1608.) |
| [19] | (h) Wang, A.; Xiao, Y.; Zhou, Y.; Xu, J.; Liu, H. Chin. J. Org. Chem. 2017, 37,2590. (in Chinese) |
| [19] | ( 王翱, 肖永龙, 周宇, 徐进宜, 柳红, 有机化学, 2017, 37,2590.) |
| [19] | (i) Qu, M.; He, J. Chin. J. Org. Chem. 2011, 31,1388. (in Chinese) |
| [19] | ( 屈孟男, 何金梅, 有机化学, 2011, 31,1388.) |
| [20] | Reddi, R.N.; Malekar, P.V.; Sudalai, A. Org. Biomol. Chem. 2013, 11,6477. |
| [21] | Reddi, R.N.; Gontala, A.; Prasad, P.K.; Sudalai, A. Asian J. Org. Chem. 2016, 5,48. |
| [22] | Forte, G.; Chiarotto, I.; Inesi, A.; Loreto, M.A.; Feroci, M. Adv. Synth. Catal. 2014, 356,1773. |
| [23] | Lu, H.; Liu, J.-Y.; Li, H.-Y.; Xu, P.-F. Acta Chim. Sinica 2018, 76,831. (in Chinese) |
| [23] | ( 鲁鸿, 刘金宇, 李红玉, 许鹏飞, 化学学报, 2018, 76,831.) |
| [24] | Nemoto, T.; Fukuda, T.; Hamada, Y. Tetrahedron Lett. 2006, 47,4365. |
| [25] | (a) Namitharan, K.; Zhu, T.; Cheng, J.; Zheng, P.; Li, X.; Yang, S.; Song, B.-A.; Chi, Y.R. Nat. Commun. 2014, 5,3982. |
| [25] | (b) Chen, J.; Yuan, P.; Wang, L.; Huang, Y. J. Am. Chem. Soc. 2017, 139,7045. |
| [26] | DiRocco, D.A.; Rovis, T. J. Am. Chem. Soc. 2012, 134,8094. |
| [27] | Chen, Z.-W.; Ye, D.-N.; Ye, M.; Zhou, Z.-G.; Li, S.-H.; Liu, L.-X. Tetrahedron Lett. 2014, 55,1373. |
| [28] | Jia, Y.; Li, T.; Yu, C.; Jiang, B.; Yao, C. Org. Biomol. Chem. 2016, 14,1982. |
| [29] | (a) Liu, Y.K.; Li, R.; Yue, L.; Li, B.J.; Chen, Y.C.; Wu, Y.; Ding, L.S. Org. Lett. 2006, 8,1521. |
| [29] | (b) Xia, Z.-H.; Dai, L.; Gao, Z.-H.; Ye, S. Chem. Commun. 2020, 56,1525. |
| [29] | (c) Gao, Z.-H.; Xia, Z.-H.; Dai, L.; Ye, S. Adv. Synth. Catal. 2020, 362,1819. |
| [30] | Liao, L.; Zhang, H.; Zhao, X. ACS Catal. 2018, 8,6745. |
| [31] | Hu, Y.; Chen, J.; Le, Z.G.; Chen, Z.C.; Zheng, Q.G. Chin. Chem. Lett. 2005, 16,903. |
/
| 〈 |
|
〉 |