

Chinese Journal of Organic Chemistry >
Triethylamine-Catalyzed Cascade Reaction of γ-Hydroxy-α,β-unsaturated Ketones with Trifluoromethyl Ketones via Hemiketal Intermediates
Received date: 2020-10-29
Revised date: 2020-12-05
Online published: 2020-12-24
Supported by
Voluntary Program of China Academy of Chinese Medical Sciences(ZZ0908028)
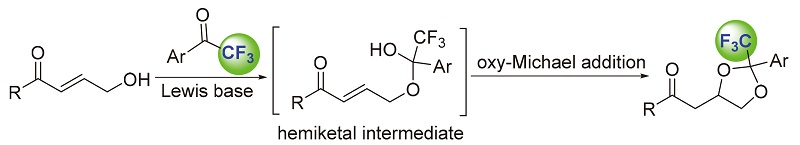
Et3N-catalyzed cascade reaction of trifluoromethyl ketones and γ-hydroxy-α,β-unsaturated ketones via hemiketal intermediate is investigated. The adducts could be easily obtained in good to excellent yields with moderate diastereoselectivities. The reaction proceeded smoothly to give the desired product in 92% yield and with 96% ee in the presence of a glycosyl and quinidine-based bifunctional chiral thiourea catalyst.
Hai Ma , Guanghao Yu , Feng Sui , Miyi Yang , Li Liu , Huijie Li , Feng Li , Qinghe Zhao . Triethylamine-Catalyzed Cascade Reaction of γ-Hydroxy-α,β-unsaturated Ketones with Trifluoromethyl Ketones via Hemiketal Intermediates[J]. Chinese Journal of Organic Chemistry, 2021 , 41(4) : 1614 -1621 . DOI: 10.6023/cjoc202010040
| [1] | (a) Royer, J. Asymmetric Synthesis of Nitrogen Heterocycles, Wiley VCH, Wienheim, 2009. |
| [1] | (b) Vo, C.-V. T.; Luescher, M.U.; Bode, J.W. Nat. Chem. 2014, 6,310. |
| [1] | (c) Yanai, H. In Green Synthetic Approaches for Biologically Relevant Heterocycles, Ed.: Brahmachari, G., Elsevier, Boston, 2015, pp.257~289. |
| [1] | (d) Meyer, A.G.; Bissember, A.C.; Hyland, C.J. T.; Williams, C.C.; Szabo, M.; Abel, S.A. G.; Bird, M.J.; Hyland, I.K.; Pham, H. In Progress in Heterocyclic Chemistry, Eds.: Gribble, G. W.; Joule, J.A., Elsevier, Amsterdam, 2018, pp.493~550. |
| [2] | (a) Yudin, A.K. Aziridines and Epoxides in Organic Synthesis, Wiley-VCH, Weinheim, 2006. |
| [2] | (b) Kannan, V.; Sreekumar, K.; Gil, A.; Vicente, M.A. Catal. Lett. 2011, 141,1118. |
| [2] | (c) Lykke, L.; Halskov, K.S.; Carlsen, B.D.; Chen, V.X.; J?rgensen, K.A. J. Am. Chem. Soc. 2013, 135,4692. |
| [3] | For selected reviews, see: (a) Aho, J. E.; Pihko, P. M.; Rissa, T. K. Chem. Rev. 2005, 105,4406. |
| [3] | (b) Carey, F.A. Organic Chemistry, 6th ed., McGraw-Hill, New York, 2006. |
| [3] | (c) Sawant, P.; Maier, M.E. Eur. J. Org. Chem., 2012,6576. |
| [3] | (d) ?ori?, I.; Vellalath, S.; Müller, S.; Cheng, X.; List, B. Top. Organomet. Chem. 2013, 44,165. |
| [3] | (e) Kim, J.H.; ?ori?, I.; Vellalath, S.; List, B. Angew. Chem., Int. Ed. 2013, 52,4474. |
| [3] | (f) Nallasivam, J.L.; Fernandes, R.A. Org. Biomol. Chem. 2017, 15,708. |
| [3] | (g) Yu, X.; Che, Z.; Xu, H. Chem.-Eur. J. 2017, 23,4467. |
| [3] | (h) Mahto, P.; Rana, N.K.; Shukla, K.; Das, B.G.; Joshi, H.; Singh, V.K. Org. Lett. 2019, 21,5962. |
| [4] | For selected reviews, see: (a) Bégué, J.-P.; Bonnet-Delpon, D.; Crousse, B.; Legros, J. Chem. Soc. Rev. 2005, 34,562. |
| [4] | (b) Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37,320. |
| [4] | (c) Xie, H.X.; Zhang, Y.; Zhan, S.L.; Cheng, X.B.; Wang, W. Angew. Chem., Int. Ed. 2011, 50,11773. |
| [4] | (d) Ma, J.-A.; Cahard, D. Chem. Rev. 2008, 108,PR1. |
| [4] | (e) Zheng, Y.; Ma, J.-A. Adv. Synth. Catal. 2010, 352,2745. |
| [4] | (f) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111,455. |
| [4] | (g) Kawai, H.; Tachi, K.; Tokunaga, E.; Shiro, M.; Shibata, N. Org. Lett. 2010, 12,5104. |
| [4] | (h) Wang, T.; Zhang, G.-W.; Teng, Y.; Nie, J.; Zheng, Y.; Ma, J.-A. Adv. Synth. Catal. 2010, 352,2773. |
| [4] | (i) Zhang, G.-W.; Meng, W.; Ma, H.; Nie, J.; Zhang, W.-Q.; Ma, J.-A. Angew. Chem., Int. Ed. 2011, 50,3538. |
| [4] | (j) Allen, A.E.; MacMillan, D.W. C. J. Am. Chem. Soc. 2010, 132,4986. |
| [4] | (k) Kawai, H.; Kitayama, T.; Tokunaga, E.; Shibata, N. Eur. J. Org. Chem. 2011, 30,5959. |
| [4] | (l) Ma, H.; Matsuzaki, K.; Yang, Y.-D.; Tokunaga, E.; Nakane, D.; Ozawa, T.; Masuda, H.; Shibata, N. Chem. Commun. 2013, 49,11206. |
| [4] | (m) Wang, J.; Sanchez-Rosello, M.; Ace?a, J.L.; Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Chem. Rev. 2014, 114,2432. |
| [5] | (a) Wang, J.; Kong, W.-G.; Li, F.; Liu, J.; Shen, Q.; Liu, L.; Zhao, W.-X. Org. Biomol. Chem. 2015, 13,5399. |
| [5] | (b) Lin, Y.-J.; Du, L.-N.; Kang, T.-R.; Liu, Q.-Z.; Chen, Z.-Q.; He, L. Chem.-Eur. J. 2015, 21,11773. |
| [5] | (c) Zhu, Y.; Dong, Z.; Cheng, X.; Zhong, X.; Liu, X.; Lin, L.; Shen, Z.; Yang, P.; Li, Y.; Wang, H.; Yan, W.; Wang, R. Org. Lett. 2016, 18,3546. |
| [6] | (a) Li, F.; Wang, J.-J.; Xu, M.-M.; Zhao, X.-B.; Zhou, X.-H.; Zhao, W.-X.; Liu, L.-T. Org. Biomol. Chem. 2016, 14,3981. |
| [6] | (b) Liliana, B.-F.; Elodie, B.; Michael, M.; Louis, J.F.; Claire, W.; Jo?lle, P.; Diego, G.-S. Org. Biomol. Chem. 2017, 15,301. |
| [7] | For reviews on oxy-Michael addition reactions,see: (a) Nising, C. F.; Brase, S. Chem. Soc. Rev. 2008, 37,1218. |
| [7] | (b) Hartmann, E.; Vyas, D.J.; Oestreich, M. Chem. Commun. 2011, 47,7917. |
| [7] | (c) Nising, C.F.; Brase, S. Chem. Soc. Rev. 2012, 41,988. |
| [8] | (a) Li, D.R.; Murugan, A.; Falck, J.R. J. Am. Chem. Soc. 2008, 130,46. |
| [8] | (b) Wang, H.F.; Yan, L.J.; Wu, Y.; Chen, F. Tetrahedron 2017, 73,2793. |
| [9] | (a) Asano, K.; Matsubara, S. Org. Lett. 2012, 14,1620. |
| [9] | (b) Okamura, T.; Asano, K.; Matsubara, S. Chem. Commun. 2012, 48,5076. |
| [9] | (c) Fukata, Y.; Miyaji, R.; Okamura, T.; Asano, K.; Matsubara, S. Synthesis 2013, 45,1627. |
| [9] | (d) Yoneda, N.; Hotta, A.; Asano, K.; Matsubara, S. Org. Lett. 2014, 16,6264. |
| [10] | (a) Fukata, Y.; Asano, K.; Matsubara, S. Chem. Lett. 2013, 42,355. |
| [10] | (b) Asano, K.; Matsubara, S. J. Am. Chem. Soc. 2011, 133,16711. |
| [10] | (c) Miyaji, R.; Asano, K.; Matsubara, S. Org. Lett. 2013, 15,3658. |
| [11] | (a) Reddy, B.V.; Reddy, S.M.; Swain, M. RSC Adv. 2013, 3,930. |
| [11] | (b) Reddy, B.V.; Reddy, S.M.; Swain, M.; Dudem, S.; Kalivendib, S.V.; Reddy, C.S. RSC Adv. 2014, 4,9107. |
/
| 〈 |
|
〉 |