

Chinese Journal of Organic Chemistry >
Desymmetrization Strategy: Synthesis of Right Segment of Ingramycin
Received date: 2020-06-29
Revised date: 2020-07-23
Online published: 2020-08-18
Supported by
the National Natural Science Foundation of China(21372205); the National Natural Science Foundation of China(21302175); the Basic Research Project of Science and Technology Department of Henan Province(132300410028)
In the construction of chiral tertiary alcohol of the natural product ingramycin, the conventional asymmetric synthesis and the method that using the chiral source as material were not used. Instead, exploring the symmetry of natural product itself, the symmetric nonchiral precursor was synthesized firstly, and then the selective ester hydrolysis reaction under lipase catalysis was carried out, which not only promoted the transformation of functional groups, but also constructed a challenging and sterically congested quaternary carbon stereocenter. Based on the readily available allyl bromide and ethyl acetate, the right segment of ingramycin was synthesized by 11 steps with total yield of 26.7% and ee value of 50.84%.
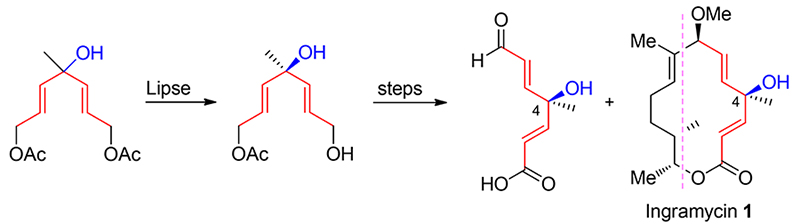
Key words: ingramycin; desymmetrization; lipase; chiral tertiary alcohol
Moran Sun , Leiyang Bai , Junhong Xiang , Hua Yang , Dequan Yu , Hongmin Liu . Desymmetrization Strategy: Synthesis of Right Segment of Ingramycin[J]. Chinese Journal of Organic Chemistry, 2021 , 41(1) : 364 -369 . DOI: 10.6023/cjoc202006067
| [1] | Schiewe H.J.; Zeeck A. J. Antibiot. 1999, 52, 635. |
| [2] | Koyama N.; Yotsumoto M.; Onaka H.; Tomoda H. J. Antibiot. 2013, 66, 303. |
| [3] | Burkhardt K.; Fiedler H.P.; Grabley S.; Thiericke R.; Zeeck A. J. Antibiot. 1996, 49, 432. |
| [4] | Schneider A.; Spath J.; BreidingMack S.; Zeeck A.; Grabley S.; Thiericke R. J. Antibiot. 1996, 49, 438. |
| [5] | Takahashi T.; Watanabe H.; Kitahara T. Tetrahedron Lett. 2003, 44, 9219. |
| [6] | Tanner D.; Somfai P. Tetrahedron 1987, 43, 4395. |
| [7] | Tietze L.-F.; Volkel L. Angew. Chem., Int. Ed. 2001, 40, 901. |
| [8] | Li G.; Yang X.; Zhai H. J. Org. Chem. 2009, 74, 1356. |
| [9] | Stephan M.; Rummelt J.-P.; Heiko S.; Alois F. Angew. Chem., Int. Ed. 2015, 54, 6241. |
| [10] | (a) Gao K.-G.; Sun M.-R.; Zhu M.; Yang H. Chin. J. Org. Chem. 2013, 33, 1939. (in Chinese) |
| [10] | ( 高凯歌, 孙默然, 朱明, 杨华, 有机化学, 2013, 33, 1939.). |
| [10] | (b) Sun M.-R.; Dai L.; Yang H.; Liu H.-M.; Yu D.-Q. Chin. J. Org. Chem. 2018, 38, 2443. (in Chinese) |
| [10] | ( 孙默然, 代磊, 杨华, 刘宏民, 于德泉, 有机化学, 2018, 38, 2443.). |
| [11] | Kerber R.C.; Hsu C.-M. J. Am. Chem. Soc. 1973, 95, 3239. |
| [12] | (a) Mickel S.J.; Sedelmeier G.H.; Niederer D. Org. Process Res. Dev. 2004, 8, 113. |
| [12] | (b) Guo R.-L.; Zhu X.-Q.; Zhang X.-L.; Wang Y.-Q. Chem. Commun. 2020, 56, 8976. |
| [12] | (c) Wang D.-Y.; Guo S.-H.; Pan G.-F.; Zhu X.-Q.; Gao Y.-R.; Wang Y.-Q. Org. Lett. 2018, 20, 1794. |
| [13] | Hwang M.-H.; Han S.-J.; Lee D.-H. Org. Lett. 2013, 15, 3318. |
| [14] | Guanti G.; Banfi L.; Narisano E. J. Org. Chem. 1992, 57, 1540. |
| [15] | (a) Laumen K.; Schneider M. Tetrahedron Lett. 1985, 26, 2073. |
| [15] | (b) Wang Y.-F.; Sih C.-J. Tetrahedron Lett. 1984, 25, 4999. |
| [15] | (c) Wang Y.-F.; Chen C.-S.; Girdaukas G. J. Am. Chem. Soc. 1984, 106, 3695. |
| [15] | (d) Arai N.; Chikaraishi N.; Ikawa M. Tetrahedron: Asymmetry 2004, 15, 733. |
| [16] | Sanchez-Larios E.; Giacometti R.D.; Hanessian S. Eur. J. Org. Chem. 2014, 26, 5664. |
| [17] | Rainer K.; Thilo B.; Reinhard B. Adv. Synth. Catal. 2008, 350, 1131. |
/
| 〈 |
|
〉 |