

Chinese Journal of Organic Chemistry >
Formylation of Phenols and Paraformaldehyde Catalyzed by Ammonium Acetate
Received date: 2020-11-09
Revised date: 2020-12-26
Online published: 2021-02-07
Supported by
National Natural Science Foundation of China(21262028); National Natural Science Foundation of China(21762039); Natural Science Foundation of Gansu Province(20JR5RA521)
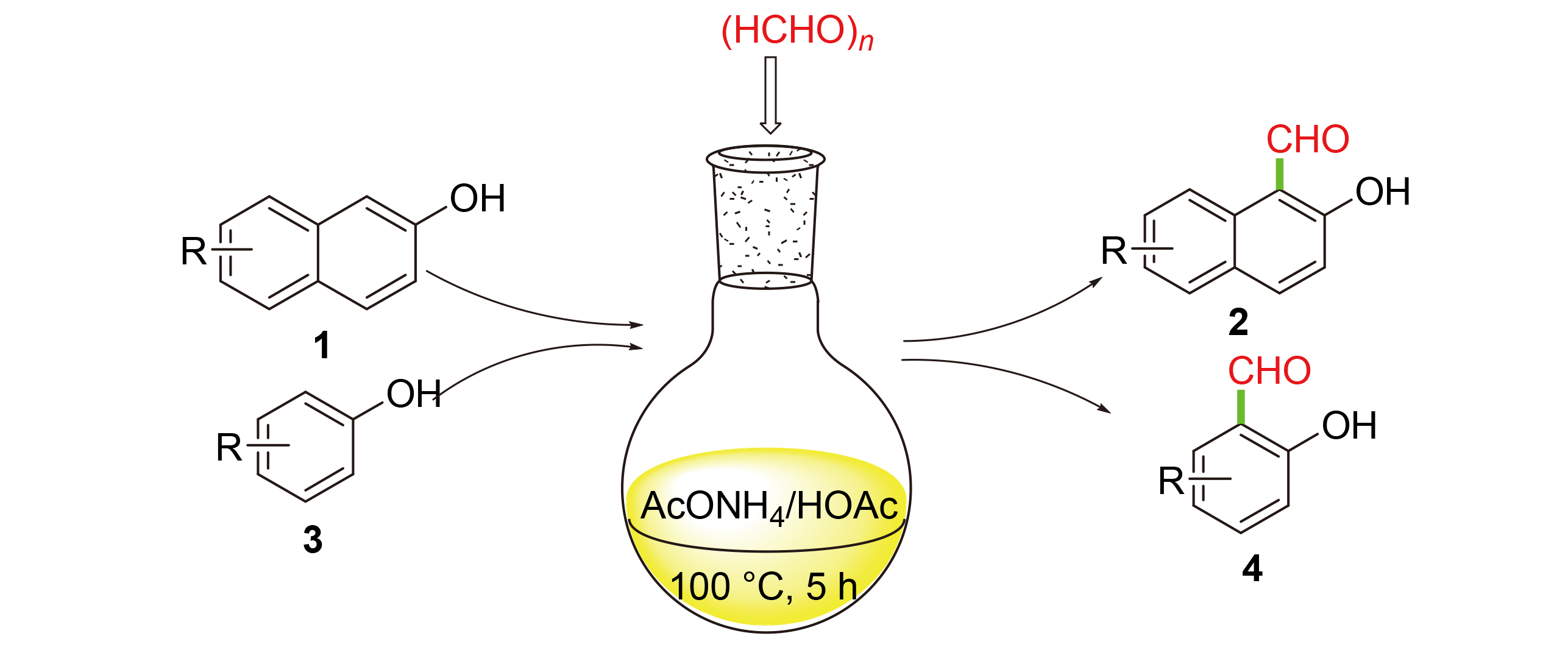
The reaction of naphthol and paraformaldehyde in acetic acid solution with ammonium acetate as a catalyst resulted in the synthesis of a series of hydroxynaphthalenes in yields of up to 86%. Several phenols were also transformed into the corresponding salicylaldehydes under the same reaction conditions in moderate yields of 42%~58%. A rational mechanism was also proposed based on the facts of experimental observations. This metal-free process has the advantages of mild reaction conditions, simple operation and low cost.
Key words: naphthol; phenol; paraformaldehyde; formylation reaction; green synthesis
Qian Li , Li Yang , Wei Liu , Tianyun Wang , Yuejie Zhu , Zhengyin Du . Formylation of Phenols and Paraformaldehyde Catalyzed by Ammonium Acetate[J]. Chinese Journal of Organic Chemistry, 2021 , 41(5) : 2038 -2044 . DOI: 10.6023/cjoc202011014
| [1] | Vojacek, S.; Beese, K.; Alhalabi, Z.; Swyter, S.; Bodtke, A.; Schulzke, C.; Jung, M.; Sippl, W.; Link, A. Arch. Pharm. Chem. Life Sci. 2017, 350, e1700097. |
| [2] | Lu, C.; Hu, J. H.; Wang, Z. C.; Xie, S. H.; Pan, T. T.; Huang, L.; Li, X. S. Med. Chem. Commun. 2018, 9, 1862. |
| [3] | Xiao, J. M.; Feng, L.; Zhou, L. S.; Gao, H. Z.; Zhang, Y. L.; Yang, K. W. Eur. J. Med. Chem. 2013, 59, 150. |
| [4] | Dobashi, Y.; Ohkatsu, Y. Polym. Degrad. Stab. 2008, 93, 436. |
| [5] | Deb, M. L.; Pegu, C. D.; Borpatra, P. J.; Baruah, P. K. RSC Adv. 2016, 6, 40552. |
| [6] | Goswami, S.; Maity, S.; Das, A. K.; Maity, A. C. Tetrahedron Lett. 2013, 54, 6631. |
| [7] | Su, H. Y.; Huang, W. W.; Yang, Z. Y.; Lin, H.; Lin, H. K. J. Inclusion Phenom. Macrocyclic Chem. 2012, 72, 221. |
| [8] | Long, F. J. Fluoresc. 2017, 27, 1331. |
| [9] | Qin, J. C.; Fan, L.; Li, T. R.; Yang, Z. Y. Synth. Met. 2015, 199, 179. |
| [10] | Ugi, I. Adv. Synth. Catal. 1997, 339, 499. |
| [11] | Terret, N. K.; Gardener, M.; Gordon, D. W.; Kobylecki, R. J.; Steele, J. Tetrahedron 1995, 51, 8135. |
| [12] | (a) Duff, J. C. J. Chem. Soc. 1941,547. |
| [12] | (b) Duff, J. C. J. Chem. Soc. 1944,276. |
| [13] | Reimer, K.; Tiemann, F. Chem. Ber. 1876, 9, 824. |
| [14] | Hofsl?kken, N. U.; Skatteb?l, L. Acta Chem. Scand. 1999, 53, 258. |
| [15] | Ma, K. R.; Huang, Z. Q.; Tang, X. D.; Zhang, P. C.; Shi, G. L. Chem. World 1987, 2, 58. (in Chinese). |
| [15] | (马克荣, 黄治清, 唐宪达, 张鹏程, 石桂林, 化学世界, 1987, 2, 58.) |
| [16] | Xia, S. P.; Lv, Y. M.; Cheng, X. Y. Sichuan Chem. Ind. 2004, 7, 10. (in Chinese). |
| [16] | (夏士朋, 吕云妹, 成新燕, 四川化工, 2004, 7, 10.) |
| [17] | Zhao, S. F.; Chen, N. Y.; Mei, S. H.; Sun, J. G.; Hao, W. D. J. Wuhan Univ. Technol. 2006, 28, 73. (in Chinese). |
| [17] | (赵胜芳, 陈年友, 梅水华, 孙国建, 郝卫东, 武汉理工大学学报, 2006, 28, 73.) |
| [18] | Yue, X. L.; Wang, Z. Q.; Li, C. R.; Yang, Z. Y. Tetrahedron Lett. 2017, 58, 4532. |
| [19] | Balali, E.; Shameli, A.; Naeimi, H.; Ghanbari, M. M. Orient. J. Chem. 2013, 29, 1611. |
| [20] | Naeimi, H.; Zakerzadeh, E. New J. Chem. 2018, 42, 4590. |
| [21] | (a) Wu, H.; Wu, J. C.; Du, Z. Chin. J. Org. Chem. 2017, 37, 1127. (in Chinese). |
| [21] | (吴慧, 武俊成, 杜正银, 有机化学, 2017, 37, 1127.) |
| [21] | (b) Hu, B.; Zhang, Y. M.; Wang, T. Y.; Du, Z. J. Liaocheng Univ. (Nat. Sci.) 2020, 33, 67. (in Chinese). |
| [21] | (胡贝, 张源民, 王天昀, 杜正银, 聊城大学学报(自然科学版), 2020, 33, 67.) |
| [21] | (c) Zhang, W.; Dong, T.; Wang, T.; Du, Z. J. Liaocheng Univ. (Nat. Sci.) 2019, 32, 35. (in Chinese). |
| [21] | (张文莹, 董涛生, 王天昀, 杜正银, 聊城大学学报(自然科学版), 2019, 32, 35.) |
| [22] | (a) Stover, J. S.; Shi, J.; Jin, W.; Vogt, P. K.; Boger, D. W. J. Am. Chem. Soc. 2009, 131, 3342. |
| [22] | (b) Aridoss, G.; Laali, K. K. J. Org. Chem. 2011, 76, 8088. |
| [23] | Ou, W.; Huang, P. Sci. China: Chem. 2020, 63, 11. |
| [24] | Yue, X.-L.; Wang, Z.-Q.; Li, C.-R.; Yang, Z.-Y. Tetrahedron Lett. 2017, 58, 4532. |
| [25] | Zhao, Q. G.; Wei, Q. C.; Xuan, M. D. Org. Lett. 2010, 12, 2202. |
| [26] | Vojacek, S.; Beese, K.; Alhalabi, Z.; Swyter, S.; Bodtke, A.; Schulzke, C.; Jung, M.; Sippl, W.; Link, A. Arch. Pharm. Chem. Life Sci. 2017, 350, e1700097. |
| [27] | Burton, H. J. Chem. Soc. 1945,280. |
| [28] | Muhammad, A.; David, O. M.; Paris, E. G. J. Chem. Soc., erkin Trans. 1 2002,1470. |
| [29] | Andrew, C.; Benniston; Jér?me, F. Tetrahedron Lett. 2008, 49, 4292. |
| [30] | Musgrave, O. C.; Skoyles, D. J. Chem. Res. 2006, 2006, 456. |
| [31] | Asthanaa, M.; Syiemliehb, I.; Kumarc, A.; Lalb, R. A. Inorg. Chim. Acta 2020, 502, 119286. |
| [32] | Li, L. J.; Fu, B.; Qiao, Y.; Wang, C.; Huang, Y. Y.; Liu, C. C.; Tian, C.; Du, J. L. Inorg. Chim. Acta 2014, 419, 135. |
| [33] | Zysman-Colman, E.; Arias, K.; Siegel, J. S. Can. J. Chem. 2009, 87, 440. |
| [34] | Balali, E.; Shameli, A.; Naeimi, H.; Ghanbari, M. Orient. J. Chem. 2013, 29, 1611. |
| [35] | Antúnez, D. J. B.; Greenhalgh, M. D.; Fallan, C.; Slawin, A. M. Z.; Smith, A. D. Org. Biomol. Chem. 2016, 14, 7268. |
| [36] | Horton, D. A.; Bourne, G. T.; Coughlan, J.; Kaiser, S. M.; Jacobs, C. M.; Jones, A.; Ruhmann, A.; Turnerb, J. Y.; Smythe, M. L. Org. Biomol. Chem. 2008, 6, 1386. |
| [37] | Knuutlnen, J. S.; Kolehmainen, E. T. J. Chem. Eng. Data 1983, 28, 139. |
| [38] | Casiraghi, G.; Casnati, G.; Puglia, G.; Sartori, G. Synthesis 1980,124. |
/
| 〈 |
|
〉 |