

Chinese Journal of Organic Chemistry >
Design, Synthesis and Anti-influenza A Virus Evaluation of Oleanolic Acid C3-Glycoconjugates
Received date: 2020-10-21
Revised date: 2020-12-15
Online published: 2021-02-22
Supported by
Science and Technology Plan Project of Yunnan Provincial Department of Science and Technology(2019FB125); Open-Fund Program of the State Key Laboratory of Natural and Biomimetic Drugs(K202003)
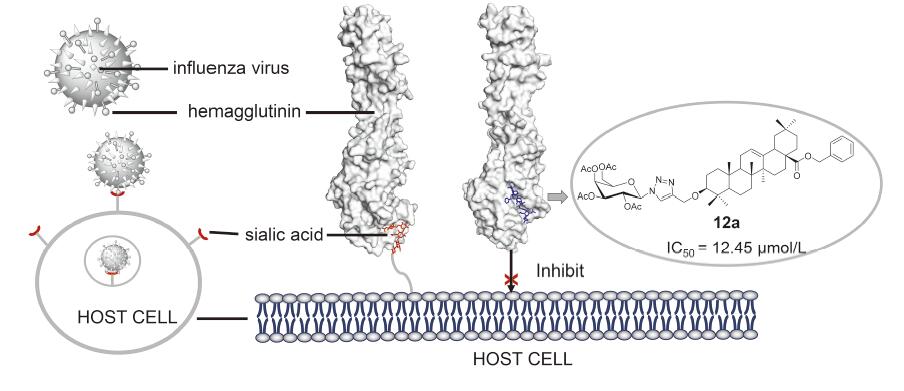
Inhibitors targeting the entry stage of influenza viruses are a hot spot in the development of anti-influenza drugs. Our previous studies showed that oleanolic acid (OA) C28 glycoconjugates displayed strong anti-influenza virus activity. In this paper, a series of oleanolic acid C3 glycoconjugates 7a~14c were designed and synthesized via copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) reaction. The anti-influenza activities of all these compounds were evaluatedin vitro. Among them, oleanane-12-enyl-28-benzyloxycarbonyl-3-O-(4-methylene-1,2,3-triazole-1-(2,3,4,6-tetra-O-acetyl-β-D-galactoside)) (12a) showed the strongest activity with an IC50 of 12.45 µmol•L –1, and no obvious cytotoxic effect on MDCK cells was observed at 100 µmol•L –1. Hemagglutination inhibition and molecular docking experiments indicated that compound 12a might target viral envelope hemagglutinin (HA), thus inhibiting the attachment of viruses to host cells. This study improved the structure-activity relationships of oleanolic acid and its derivatives against influenza virus, and provided a basis for further research on anti-virus by these natural products.
Key words: oleanolic acid; glycoconjugates; influenza virus; CuAAC reaction; inhibitors
Liang Shao , Fan Yang , Weijia Li , Fei Yu . Design, Synthesis and Anti-influenza A Virus Evaluation of Oleanolic Acid C3-Glycoconjugates[J]. Chinese Journal of Organic Chemistry, 2021 , 41(6) : 2454 -2466 . DOI: 10.6023/cjoc202010029
| [1] | CDC. Influenza Type A Viruses[EB/OL]. [2017-04-19]. https: //www. cdc. gov/flu/avianflu/influenza-a-virus-subtypes. htm |
| [2] | Kilbourne; Edwin, D. Emerg Infect Dis. 2006, 12(1),9. |
| [3] | van Dongen, M.; Kadam, R. U.; Juraszek, J.; Lawson, E.; Brandenburg, B.; Schmitz, F.; Schepens, W.; Stoops, B.; van Diepen, H. A.; Jongeneelen, M.; Tang, C.; Vermond, J.; van Eijgen-Obregoso Real, A.; Blokland, S.; Garg, D.; Yu, W.; Goutier, W.; Lanckacker, E.; Klap, J. M.; Peeters, D.; Wilson, I. A. Science. 2019, 363(6431),6221. |
| [4] | Fang, Y.; Xiao, M.; Hu, A.; Ye, J.; Lian, W.; Liu, A. Chin J. Chem. 2016, 34,403. |
| [5] | Sheu, T. G.; Fry, A. M.; Garten, R. J.; Deyde, V. M.; Shwe, T.; Bullion, L.; Peebles, P. J.; Li, Y.; Klimov, A. I.; Gubareva, L. V. J Infect Dis. 2011, 203(1),13. |
| [6] | Hay, A. J.; Hayden, F. G. Lancet. 2013, 381(9885),2230. |
| [7] | Heo, Y. A. Drugs. 2018, 78(6),693. |
| [8] | Hayden, F. G.; & Shindo, N. Curr Opin Infect Dis. 2019, 32(2),176. |
| [9] | Vanderlinden, E.; Naesens, L. Med. Res. Rev. 2014, 34,301. |
| [10] | Zhao, X.; Li, R.; Zhou, Y.; Xiao, M.; Ma, C.; Yang, Z.; Zeng, S.; Du, Q.; Yang, C.; Jiang, H.; Hu, Y.; Wang, K.; Mok, C K P.; Sun, P.; Dong, J.; Cui, W.; Wang, J.; Tu, Y.; Yang, Z.; Hu, W. J Med Chem. 2018, 61(12),5187. |
| [11] | Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Yu, F.; Jiao, P.; Shi, Y.; Wang, H.; Xiao, S.; Fu, G.; Tian, K.; Wang, Y.; Guo, Z.; Ye, X.; Zhang, L.; Zhou, D. J. Med. Chem. 2014, 57,10058. |
| [12] | Li, S.; Jia, X.; Shen, X.; Wei, Z.; Jiang, Z.; Liao, Y.; Guo, Y.; Zheng, X.; Zhong, G.; Song, G. Bioorg Med Chem. 2017, 25(16),4384. |
| [13] | Song, G.; Shen, X.; Li, S.; Li, Y.; Si, H.; Fan, J.; Li, J.; Gao, E.; Liu, S. Eur J Med Chem. 2016, 119,109. |
| [14] | Song, G.; Shen, X.; Li, S.; Si, H.; Li, Y.; Luan, H.; Fan, J.; Liang, Q.; Liu, S. RSC Adv. 2015, 5,39145. |
| [15] | Su, Y.; Meng, L.; Sun, J.; Li, W.; Shao, L.; Chen, K.; Zhou, D.; Yang, F.; Yu, F. Eur J Med Chem. 2019, 182,111622. |
| [16] | Conte, G.; Cristiano, R.; Ely, F.; Gallardo, H. Synth. Commun. 2006, 36,951. |
| [17] | Chittaboina, S.; Xie, F., Wang, Q. (2005). Tetrahedron Letters 2005, 46(29),2331. |
| [18] | Maier, M. A.; Yannopoulos, C. G.; Mohamed, N.; Roland, A.; Fritz, H.; Mohan, V.; Just, G.; Manoharan, M. Bioconjug Chem. 2003, 14(1),18. |
| [19] | Zhao, S.; Zhang, S.; Xu, J.; Hu, L. Tetrahedron. 2020, 76(42),131517. |
| [20] | Tang, Y.; Zhang, S.; Chang, Y.; Fan, D.; Agostini, A.; Zhang, L.; Jiang, T. J Med Chem. 2018, 61(7),2937. |
| [21] | Andreeva, O.; Garifullin, B.; Sharipova, R.; Strobykina, I.; Sapunova, A.; Voloshina, A.; Belenok, M.; Dobrynin, A.; Khabibulina, L.; Kataev, V. J Nat Prod. 2020, 83(8),2367. |
| [22] | Tsoulougian, V.; Psykarakis, E.; Gimisis, T. Org Biomol Chem. 2019, 17(4),973. |
| [23] | Novoa, A.; Machida, T.; Barluenga, S.; Imberty, A.; Winssinger, N. Chembiochem. 2015, 15(14),2058. |
| [24] | Yu, F.; Zheng, Y.; Peng, Y.; Zhou, D.; Xiao, S. Design and synthesis of oleanolic acid rings a and c lactone derivatives. Chin. J. Org. Chem, 2016, 36,512(in Chinese). |
| [24] | (俞飞, 郑永祥, 彭逸云, 周德敏, 肖苏龙, 有机化学, 2016, 36,512.) |
| [25] | Li, W.; Yang, F.; Meng, L.; Sun, J.; Su, Y.; Shao, L.; Zhou, D.; Yu, F. Chem. Pharm Bull. 2019, 67(11),1201. |
/
| 〈 |
|
〉 |