

Chinese Journal of Organic Chemistry >
Synthesis, Herbicidal Activity and Three-Dimensional Quantitative Structure-Activity Relationship (3D-QSAR) Study of 4-Methyl- 1,2,4-triazole-thioether Compounds Containing Natural Styrene Structure
Received date: 2020-11-17
Revised date: 2020-12-26
Online published: 2021-02-22
Supported by
National Natural Science Foundation of China(31870556)
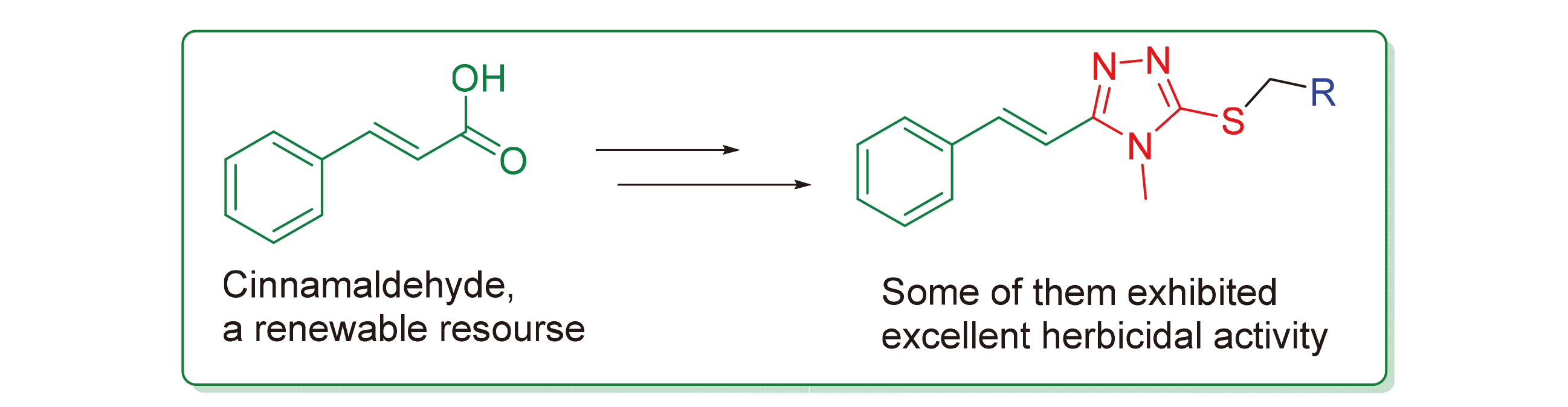
In an attempt to search for natural renewable resource-based herbicidal agents, twenty-six novel 4-methyl-1,2,4- triazole-thioether compounds containing natural styrene structure were designed and synthesized. Their structures were confirmed by FTIR,1H NMR, 13C NMR, ESI-MS and elemental analysis. The preliminary herbicidal activity test showed that, at 100 µg/mL, most of the compounds showed good inhibitory activity against the root-growth of rape ( Brassica campestris), in which 8 compounds had inhibition rate of greater than 81.4%, implying much better herbicidal activity than that of the positive control flumioxazin with inhibition rate of 63.0%. Also, 2 compounds exhibited good inhibitory activity against the seedling-growth of barnyardgrass (Echinochloa crusgalli). It was found by comparison that aliphatic R substituents or pyridine rings were beneficial to herbicidal activity. Furthermore, a preliminary three-dimensional quantitative structure-activity relationship (3D-QSAR) study was carried out by the CoMFA method for the inhibitory activity of the target compounds with aromatic R substituents against the root-growth of rape, and a reasonable and effective 3D-QSAR model (r2=0.996, q2=0.603) has been established.
Chengfei Li , Bo Cen , Wengui Duan , Guishan Lin , Xiu Wang , Baoyu Li . Synthesis, Herbicidal Activity and Three-Dimensional Quantitative Structure-Activity Relationship (3D-QSAR) Study of 4-Methyl- 1,2,4-triazole-thioether Compounds Containing Natural Styrene Structure[J]. Chinese Journal of Organic Chemistry, 2021 , 41(6) : 2485 -2495 . DOI: 10.6023/cjoc202011023
| [1] | Yang, L.; Zhou, L. Z.; Chen, H. Y.; Gu, Y. Farm. Prod. Process 2019, 2,41(in Chinese). |
| [1] | ( 杨漓, 周丽珠, 陈海燕, 谷瑶, 农产品加工, 2019, 2,41. ) |
| [2] | Wu, C. H.; Shu, M.; Li, Q.; Ding, P. Chin. Med. J. Res. Prac. 2017, 31,14(in Chinese). |
| [2] | ( 伍彩红, 舒眉, 李倩, 丁平, 现代中药研究与实践, 2017, 31,14.) |
| [3] | Li, Q. Z.; Song, B. A.; Cai, X. J.; Zheng, Y. G.; Guo, Q. Q. Chin. J. Org. Chem. 2010, 30,569(in Chinese). |
| [3] | ( 李黔柱, 宋宝安, 蔡学建, 郑玉国, 郭晴晴, 有机化学, 2010, 30,569.) |
| [4] | Zhang, X. B.; Ma, H. Y.; Sun, T. D.; Lei, P.; Yang, X. L.; Zhang, X. M.; Ling, Y. Chin. J. Org. Chem. 2019, 39,2965(in Chinese). |
| [4] | ( 张学博, 马航宇, 孙腾达, 雷鹏, 杨新玲, 张晓鸣, 凌云, 有机化学, 2019, 39,2965.) |
| [5] | Marona, H.; Szkaradek, N.; Karczewska, E.; Trojanowska, D.; Budak, A.; Bober, P.; Przepiorka, W.; Marek Cegla, M.; Szneler, E. Arch. Pharm. Chem. Life Sci. 2010, 342,9. |
| [6] | Xu, Y.; Lei, P.; Ling, Y.; Wang, S. W.; Yang, X. L. Chin. J. Org. Chem. 2014, 34,1118(in Chinese). |
| [6] | ( 徐焱, 雷鹏, 凌云, 王圣文, 杨新玲, 有机化学, 2014, 34,1118.) |
| [7] | Friedman, M. J. Agric. Food Chem. 2017, 65,10406. |
| [8] | Li, X.; sheng, J. Z.; Huang, G. H.; Ma, R. X.; Yin, F. X.; Song, D.; Zhao, C.; Ma, S. T. Eur. J. Med. Chem. 2015, 97,32. |
| [9] | Kim, H. K.; Kim, J. R.; Ahn, Y. J. J. Stored. Prod. Res. 2004, 40,55. |
| [10] | Saad, M. M. G.; Gouda, N. A. A.; Abdelgaleil, S. A. M. J. Environ. Sci. Heal. B 2019, 54,954. |
| [11] | Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Molecules 2018, 23,471. |
| [12] | Muñoz, M.; Torres‐Pagán, N.; Peiró, R.; Guijarro, R.; Sánchez‐Moreiras, A. M.; Verdeguer, M. Agronomy 2020, 10,791. |
| [13] | Zhou, J.; Yuan, X. R.; Li, L.; Zhang, T.; Wang, B. Nat. Prod. Res. 2017, 31,2909. |
| [14] | Kwon, B. M.; Lee, S. H.; Choi, S. U.; Park, S. H.; lee, C. O.; Cho, Y. K.; Sung, N. D.; Bok, S. H. Arch. Pharm. Res. 1998, 21,147. |
| [15] | Ka, H.; Park, H. J.; Jung, H. J.; Choi, J. W.; Cho, K. S.; Ha, J.; Lee, K. T. Cancer Lett. 2003, 196,143. |
| [16] | Cabello, C. M.; 3rd, W. B. B.; Lamore, S. D.; Ley, S.; Bause, A. S.; Azimian, S.; Wondrak, G.T.. Free Radic. Biol. Med. 2009, 46,220. |
| [17] | Wu, W. N.; Jiang, Y. M.; Zhang, B. Y.; Wang, J. Z.; Du, H. T.; Fei, Q. Chemistry 2019, 82,1115(in Chinese). |
| [17] | ( 吴文能, 姜阳明, 张卜艳, 王家忠, 杜海堂, 费强, 化学通报, 2019, 82,1115.) |
| [18] | He, S. C.; Zhang, H. Z.; Zhang, H. J.; Sun, Q.; Zhou, C. H. Med. Chem. 2020, 16,104. |
| [19] | Liu, X. H.; Sun, Z. H.; Yang, M. Y.; Tan, C. X.; Weng, J. Q.; Zhang, Y. G.; Ma, Y. Chem. Biol. Drug Des. 2014, 84,342. |
| [20] | Rode, N. D.; Sonawane, A. D.; Nawale, L.; Khedkar, V. M.; Joshi, R. A.; Likhite, A. P.; Sarkar, D.; Joshi, R. R. Chem. Biol. Drug Des. 2017, 90,1206. |
| [21] | Gilandoust, M.; Harsha, K. B.; Mohan, C. D.; Raquib, A. R.; Rangappa, S.; Pandey, V.; Lobie, P. E.; Basappa |
| [22] | Korcz, M.; Saczewski, F.; Bednarski, P. J.; Kornicka, A. Molecules 2018, 23,1497. |
| [23] | Massari, S.; Nannetti, G.; Desantis, J.; Muratore, G.; Sabatini, S.; Manfroni, G.; Mercorelli, B.; Cecchetti, V.; Palu, G.; Cruciani, G.; Loregian, A.; Goracci, L.; Tabarrini, O. J. Med. Chem. 2015, 58,3830. |
| [24] | Liu, X. H.; Xu, X. Y.; Tan, C. X.; Weng, J. Q.; Xin, J. H.; Chen, J. Pest Manage. Sci. 2015, 71,292. |
| [25] | Wang, B. L.; Zhang, L. Y.; Liu, X. H.; Ma, Y.; Zhang, Y.; Li, Z. M.; Zhang, X. Bioorg. Med. Chem. Lett. 2017, 27,5457. |
| [26] | Wang, B. L.; Zhang, L. Y.; Zhan, Y. Z.; Zhang, Y.; Zhang, X.; Wang, L. Z.; Li, Z. M. J. Fluorine Chem. 2016, 184,36. |
| [27] | Xu, W. M.; Song, B. A.; Yang, S.; Hu, D. Y.; Zeng, S. Agrochemicals 2010, 49,625(in Chinese). |
| [27] | ( 徐维明, 宋宝安, 杨松, 胡德禹, 曾松, 农药, 2010, 49,625.) |
| [28] | HRAC Classification on Mode of Action 2020. (accessed September 26, 2020). https://www.hracglobal.com. |
| [29] | Lu, X. L.; Zhu, X.; Zhang, M.; Wu, Q. L.; Zhou, X. D.; Li, J. K. Nat. Prod. Lett. 2019, 33,2145. |
| [30] | Cheng, C. R.; Zheng, Z.; Liang, R. M.; Li, F.; X. Jiang, Q. Q.; Yue, L.; Wang, Q.; Ding, J.; Liu, Y.. Chem. Nat. Compd. 2020, 56,264. |
| [31] | Chen, Y.; Li, P.; Chen, M.; He, J.; Su, S. J.; He, M.; Wang, H.; Xue, W. J. Heterocycl. Chem. 2020, 57,1. |
| [32] | Ruan, X. H.; Zhang, C.; Jiang, S. C.; Guo, T.; Xia, R. J.; Chen, Y.; Tang, X.; Xue, W. Molecules 2018, 23,3132. |
| [33] | Barrows, R. D.; Hammill, J. T.; Michael, C. Tran, M. C.; Falade, M. O.; Rice, A. L.; Davis, C. W.; Emge, T. J.; Rablen, P. R.; Guy, R. K.; Knapp, S. Bioorg. Med. Chem. 2020 , 28, 115758.. |
| [34] | Guo, Y.; Wang, X. G.; Fan, J. P.; Zhang, Q.; Wang, Y.; Zhao, Y.; Huang, M. X.; Ding, M.; Zhang, Y. B. Roy. Soc. Open Sci. 2017, 4,171053. |
| [35] | Yu, Y. P.; Duan, W. G.; Lin, G. S.; Kang, G. Q.; Wang, X. Y.; Lei, F. H. Chin. J. Org. Chem. 2020, 40,1647(in Chinese). |
| [35] | ( 虞友培, 段文贵, 林桂汕, 康国强, 王晓宇, 雷福厚, 有机化学, 2020, 40,1647.) |
| [36] | Li, F. Y.; Huang, L.; Zhou, X. Q.; Li, Q.; Ma, X. L.; Duan, W. G.; Wang, X. Chin. J. Org. Chem. 2020, 40,2845(in Chinese). |
| [36] | ( 李芳耀, 黄琳, 周小群, 李倩, 马献力, 段文贵, 王秀, 有机化学, 2020, 40,2845.) |
| [37] | Lin, G. S.; Chen, Z. C.; Duan, W. G.; Wang, X. Y.; Lei, F. H. Chin. J. Org. Chem. 2018, 38,2085(in Chinese). |
| [37] | ( 林桂汕, 陈智聪, 段文贵, 王晓宇, 雷福厚, 有机化学, 2018, 38,2085.) |
| [38] | He, Y.; Duan, W. G.; Lin, G. S.; Cen, B.; Bu, J. W.; Lei, F. H. Chem. Ind. Forest Prod. 2020, 40,76(in Chinese). |
| [38] | ( 何云, 段文贵, 林桂汕, 岑波, 卜俊文, 雷福厚, 林产化学与工业, 2020, 40,76.) |
| [39] | Lin, G. S.; Bai, X.; Duan, W. G.; Cen, B.; Huang, M.; Lu, S. Z. ACS Sustainable Chem. Eng. 2019, 7,7862. |
| [40] | Lin, G. S.; Duan, W. G.; Yang, L. X.; Huang, M.; Lei, F. H. Molecules 2017, 22,193. |
| [41] | Verma, J.; Khedkar, V. M.; Coutinho, E. C. Curr. Top. Med. Chem. 2010, 10,95. |
| [42] | Li, L. H.; Li, Z. R.; Liu, M. L.; Shen, W. Y.; Wang, B.; Guo, H. Y.; Lu, Y. Molecules 2015, 21,49. |
| [43] | Liu, M.X; Li, C. J.. Angew. Chem. Int. Ed. 2016, 55,10806. |
| [44] | Li, L. H.; Li, Z. R.; Liu, M. L.; Shen, W. Y.; Wang, B.; Guo, H. Y.; Lu, Y. Molecules 2016, 21,49. |
| [45] | Su, N. N.; Li, Y.; Yu, S. J.; Zhang, X.; Liu, X. H.; Zhao, W. G. Res. Chem. Intermediat. 2012, 39,759. |
/
| 〈 |
|
〉 |