

Chinese Journal of Organic Chemistry >
Nickel-Catalyzed Multicomponent Coupling of Butadiene, Aldehydes, Alkynes and Schwartz Reagent to Form 1,4-Dienes
Received date: 2021-01-13
Revised date: 2021-01-28
Online published: 2021-02-22
Supported by
National Natural Science Foundation of China(21690074); National Natural Science Foundation of China(21871288); National Natural Science Foundation of China(91856111); National Natural Science Foundation of China(21821002); Strategic Priority Research Program of the Chinese Academy of Sciences(XDB20000000)
The construction of skipped diene is a vital research area for organic synthesis, whose structure is found in many bioactive molecules. The synthesis of skipped diene from simple and readily available starting materials is highly desirable. Herein a nickel-catalyzed multicomponent coupling of 1,3-butadiene, aldehydes, alkynes, and Schwartz reagents for the preparation of skipped dienes is described. The reagents are common feedstock chemicals, especially 1,3-butadiene is an abundant feedstock produced from petroleum cracking. Moreover, the hydrozirconation of alkynes using Schwartz reagent was applied to in-situ prepared the alkenylzirconium reagents, which were used directly without further treatment. Various (E,E)-1,4-diene products were synthesized with excellent regio- and stereo-selectivity. The mild and straightforward reaction condition enables a broad substrate scope and good functional group tolerance. This protocol provides a useful and practical synthesis of skipped dienes.
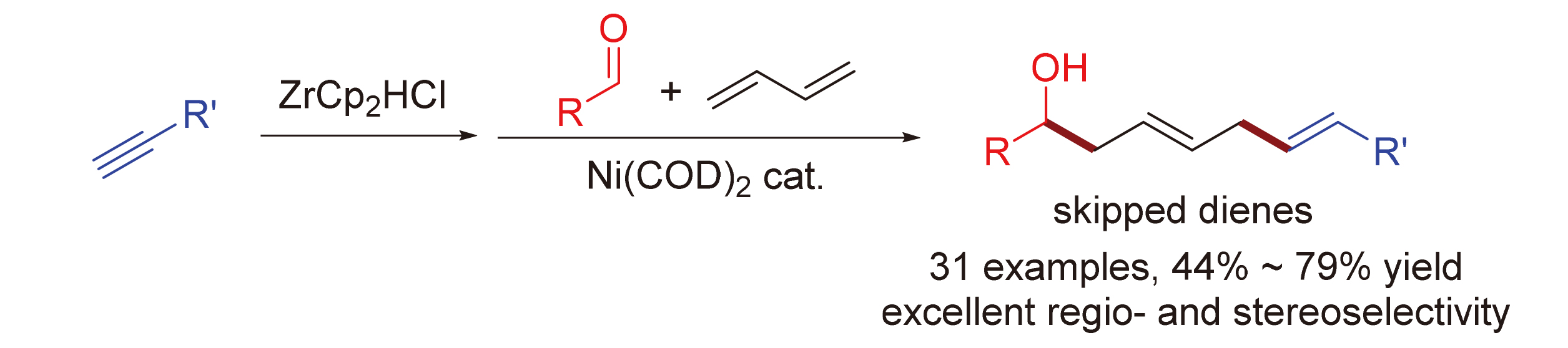
Key words: skipped diene; nickel catalysis; butadiene; multicomponent reaction; coupling
Yu-Qing Li , Shi-Liang Shi . Nickel-Catalyzed Multicomponent Coupling of Butadiene, Aldehydes, Alkynes and Schwartz Reagent to Form 1,4-Dienes[J]. Chinese Journal of Organic Chemistry, 2021 , 41(5) : 1939 -1948 . DOI: 10.6023/cjoc202101019
| [1] | (a) Winter, P.; Hiller, W.; Christmann, M. Angew. Chem., Int. Ed. 2012, 51, 3396. |
| [1] | (b) Tang, W.; Prusov, E. V. Angew. Chem., Int. Ed. 2012, 51, 3401. |
| [1] | (c) Shin, J.; Paul, V. J.; Fenical, W. Tetrahedron Lett. 1986, 27, 5189. |
| [1] | (d) Shinohara, Y.; Kudo, F.; Eguchit, T. J. Am. Chem. Soc. 2011, 133, 18134. |
| [2] | (a) Roulet, J. M.; Deguin, B.; Vogel, P. J. Am. Chem. Soc. 1994, 116, 3639. |
| [2] | (b) Denmark, S. E.; Guagnano, V.; Dixon, J. A.; Stolle, A. J. Org. Chem. 1997, 62, 4610. |
| [2] | (c) Wang, P.-S.; Liu, P.; Zhai, Y.-J.; Lin, H.-C.; Han, Z.-Y.; Gong, L.-Z. J. Am. Chem. Soc. 2015, 137, 12732. |
| [2] | (d) Lin, H.-C.; Wang, P.-S.; Tao, Z.-L.; Chen, Y.-G.; Han, Z.-Y.; Gong, L.-Z. J. Am. Chem. Soc. 2016, 138, 14354. |
| [2] | (e) Zhou, X.-L.; Su, Y.-L.; Wang, P.-S.; Gong, L.-Z. Acta Chim. Sinica 2018, 76, 857. (in Chinese). |
| [2] | (周霄乐, 苏永亮, 汪普生, 龚流柱, 化学学报, 2018, 76, 857.) |
| [2] | (f) Lin, H.-C.; Xie, P.-P.; Dai, Z.-Y.; Zhang, S.-Q.; Wang, P.-S.; Chen, Y.-G.; Wang, T.-C.; Hong, X.; Gong, L.-Z. J. Am. Chem. Soc. 2019, 141, 5824. |
| [2] | (g) Fan, L.-F.; Luo, S.-W.; Chen, S.-S.; Wang, T.-C.; Wang, P.-S.; Gong, L.-Z. Angew. Chem., Int. Ed. 2019, 58, 16806. |
| [3] | (a) Sturla, S. J.; Kablaoui, N. M.; Buchwald, S. L. J. Am. Chem. Soc. 1999, 121, 1976. |
| [3] | (b) Snider, B. B. Acc. Chem. Res. 1980, 13, 426. |
| [4] | (a) Schnermann, M. J.; Romero, F. A.; Hwang, I.; Nakamaru-Ogiso, E.; Yagi, T.; Boger, D. L. J. Am. Chem. Soc. 2006, 128, 11799. |
| [4] | (b) Pospís?il, J.; Marko?, I. E. J. Am. Chem. Soc. 2007, 129, 3516. |
| [4] | (c) Liu, W. B.; He, H.; Dai, L. X.; You, S. L. Chem.-Eur. J. 2010, 16, 7376. |
| [4] | (d) Lin, A.; Wang, J.; Mao, H.; Shi, Y.; Mao, Z.; Zhu, C. Eur. J. Org. Chem. 2013,6241. |
| [4] | (e) Ma, X.-T.; Wang, Y.; Dai, R.-H.; Liu, C.-R.; Tian, S.-K. J. Org. Chem. 2013, 78, 11071. |
| [4] | (f) Xu, S.; Zhu, S.; Shang, J.; Zhang, J.; Tang, Y.; Dou, J. J. Org. Chem. 2014, 79, 3696. |
| [5] | (a) Basavaiah, D.; Kumaragurubaran, N.; Sharada, D. S. Tetrahedron Lett. 2001, 42, 85. |
| [5] | (b) Basavaiah, D.; Sharada, D. S.; Kumaragurubaran, N.; Reddy, R. M. J. Org. Chem. 2002, 67, 7135. |
| [6] | (a) Hamilton, J. Y.; Sarlah, D.; Carreira, E. M. J. Am. Chem. Soc. 2013, 135, 994. |
| [6] | (b) McCammant, M. S.; Liao, L.; Sigman, M. S. J. Am. Chem. Soc. 2013, 135, 4167. |
| [6] | (c) Li, H.; Zhang, Z.; Shangguan, X.; Huang, S.; Chen, J.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2014, 53, 11921. |
| [6] | (d) Bin, H.-Y.; Wei, X.; Zi, J.; Zuo, Y.-J.; Wang, T.-C.; Zhong, C. M. ACS Catal. 2015, 5, 6670. |
| [6] | (e) Xu, G.; Zhao, H.; Fu, B.; Cang, A.; Zhang, G.; Zhang, Q.; Xiong, T.; Zhang, Q. Angew. Chem., Int. Ed. 2017, 56, 13130. |
| [6] | (f) Song, F.; Wang, F.; Guo, L.; Feng, X.; Zhang, Y.; Chu, L. Angew. Chem., Int. Ed. 2020, 59, 177. |
| [6] | (t) Li, W.; Yu, S.; Li, J.; Zhao, Y. Angew. Chem., Int. Ed. 2020, 59, 14404. |
| [7] | (a) RajanBabu, T. V. Synlett 2009,853. |
| [7] | (b) Hilt, G.; du Mesnil, F.-X.; Lu?ers, S. Angew. Chem., Int. Ed. 2001, 40, 387. |
| [7] | (c) Jing, S. M.; Balasanthiran, V.; Pagar, V.; Gallucci, J. C.; RajanBabu, T. V. J. Am. Chem. Soc. 2017, 139, 18034. |
| [7] | (d) Lian, X.; Chen, W.; Dang, L.; Li, Y.; Ho, C.-Y. Angew. Chem., Int. Ed. 2017, 56, 9048. |
| [7] | (e) Schmidt, V. A.; Kennedy, C. R.; Bezdek, M. J.; Chirik, P. J. J. Am. Chem. Soc. 2018, 140, 3443. |
| [8] | (a) Kimura, M.; Ezoe, A.; Mori, M.; Tamaru, Y. J. Am. Chem. Soc. 2005, 127, 201. |
| [8] | (b) Kimura, M.; Kojima, K.; Tatsuyama, Y.; Tamaru, Y. J. Am. Chem. Soc. 2006, 128, 6332. |
| [9] | (a) McCammant, M. S.; Liao, L.; Sigman, M. S. J. Am. Chem. Soc. 2013, 135, 4167. |
| [9] | (b) McCammant, M. S.; Sigman, M. S. Chem. Sci. 2015, 6, 1355. |
| [10] | For selected reviews on difunctionalization of 1,3-dienes, see: (a) Wu, X.; Gong, L.-Z. Synthesis 2019, 51, 122. |
| [10] | (b) Xiong, Y.; Sun, Y. W.; Zhang, G.-Z. Tetrahedron Lett. 2018, 59, 347. |
| [10] | (c) Wu, Z.; Zhang, W. Chin. J. Org. Chem. 2017, 37, 2250. |
| [10] | For selected examples, see:. |
| [10] | (d) Luo, Y.-C.; Xu, C.; Zhang, X. Chin. J. Chem. 2020, 38, 1371. |
| [10] | (e) Gui, Y.-Y.; Hu, N.; Chen, X.-W.; Liao, L.-L.; Ju, T.; Ye, J.-H.; Zhang, Z.; Li, J.; Yu, D.-G. J. Am. Chem. Soc. 2017, 139, 17011. |
| [10] | (f) Fu, B.; Yuan, X.; Li, Y.; Wang, Y.; Zhang, Q.; Xiong, T.; Zhang, Q. Org. Lett. 2019, 21, 3576. |
| [10] | (g) Xiong, Y.; Zhang, G. J. Am. Chem. Soc. 2018, 140, 2735. |
| [10] | (h) Tao, Z. L.; Adili, A.; Shen, H. C.; Han, Z. Y.; Gong, L. Z. Angew. Chem., Int. Ed. 2016, 55, 4322. |
| [10] | (i) Yang, J.; Ji, D. W.; Hu, Y. C.; Min, X. T.; Zhou, X.; Chen, Q. A. Chem. Sci. 2019, 10, 9560. |
| [10] | (j) Xiao, L. J.; Cheng, L.; Feng, W. M.; Li, M. L.; Xie, J. H.; Zhou, Q. L. Angew. Chem., Int. Ed. 2018, 57, 461. |
| [11] | (a) Holmes, M.; Schwartz, L. A.; Krische, M. J. Chem. Rev. 2018, 118, 6026. |
| [11] | (b) Nguyen, K. D.; Park, B. Y.; Luong, T.; Sato, H.; Garza, V. J.; Krische, M. J. Science 2016, 354, ahh5133. |
| [11] | (c) Montgomery, L. Angew. Chem., Int. Ed. 2004, 43, 3890. |
| [11] | (d) Doerksen, R. S.; Meyer, C. C.; Krische, M. J. Angew. Chem., Int. Ed. 2019, 58, 14055. |
| [11] | (e) Standley, E. A.; Tasker, S. Z.; Jensen, K. L.; Jamison, T. F. Acc. Chem. Res. 2015, 48, 1503. |
| [11] | (d) Hoshimoto, Y.; Ohashi, M.; Ogoshi, S. Acc. Chem. Res. 2015, 48, 1746. |
| [12] | (a) Zhang, W.-B.; Yang, X.-T.; Ma, J.-B.; Su, Z.-M.; Shi, S.-L. J. Am. Chem. Soc. 2019, 141, 5628. |
| [12] | (b) Cai, Y.; Zhang, J.-W.; Li, F.; Liu, J.-M.; Shi, S.-L. ACS Catal. 2019, 9, 1. |
| [12] | (c) Cai, Y.; Ye, X.; Liu, S.; Shi, S.-L. Angew. Chem., Int. Ed. 2019, 58, 13433. |
| [12] | (d) Shen, D.; Zhang, W.-B.; Li, Z.; Shi, S.-L.; Xu, Y. Adv. Synth. Catal. 2020, 362, 1125. |
| [12] | (e) Li, Y.-Q.; Li, F.; Shi, S.-L. Chin. J. Chem. 2020, 38, 1035. |
| [13] | (a) Yang, Y.; Zhu, S.-F.; Duan, H.-F.; Zhou, C.-Y.; Wang, L.-X.; Zhou, Q.-L. J. Am. Chem. Soc. 2007, 129, 2248. |
| [13] | (b) Sato, Y.; Takimoto, M.; Hayashi, K.; Katsuhara, T.; Takagi, K.; Mori, M. J. Am. Chem. Soc. 1994, 116, 9771. |
| [13] | (c) Kimura, M.; Ezoe, A.; Shibata, K.; Tamaru, Y. J. Am. Chem. Soc. 1998, 120, 4033. |
| [13] | (d) Kimura, M.; Fujimatsu, H.; Ezoe, A.; Shibata, K.; Shimizu, M.; Matsumoto, S.; Tamaru, Y. Angew. Chem., Int. Ed. 1999, 38, 397. |
| [13] | (e) Kimura, M.; Ezoe, A.; Tanaka, S.; Tamaru, Y. Angew. Chem., Int. Ed. 2001, 40, 3600. |
| [13] | (f) Kimura, M.; Ezoe, A.; Mori, M.; Iwata, K.; Tamaru, Y. J. Am. Chem. Soc. 2006, 128, 8559. |
| [13] | (g) Loh, T.-P.; Song, H. Y.; Zhou, Y. Org. Lett. 2002, 4, 2715. |
| [13] | (h) Sato, Y.; Hinata, Y.; Seki, R.; Oonishi, Y.; Saito, N. Org. Lett. 2007, 9, 5597. |
| [13] | (i) Cho, H. Y.; Morken, J. P. J. Am. Chem. Soc. 2010, 132, 7576. |
| [13] | (j) Ogoshi, S.; Tonomori, K.; Oka, M. A. J. Am. Chem. Soc. 2006, 128, 7077. |
| [13] | (k) Li, Y.-L.; Li, W.-D.; Gu, Z.-Y.; Chen, J.; Xia, J.-B. ACS Catal. 2019, 10, 1528. |
| [13] | (l) Chen, B.; Zhang, Y.; Wu, Y.; Fang, D.; Chen, X.; Wang, S.; Zhao, Y.; Hu, P.; Zhao, K.-Q.; Wang, B.-Q.; Cao, P. ACS Catal. 2019, 9, 11788. |
| [14] | Song, Z.; Takahashi, T. Hydrozirconation of Alkenes and Alkynes. Hydrozirconation of Alkenes and Alkynes, In Comprehensive Organic Synthesis II, 2 ed., Ed.: Knochel, P., Elsevier, London, 2014, pp.838~876. |
| [15] | During our manuscript preparation, similar work was reported, which utilize aldehydes, dienes and isolated vinylzirconium reagents to afford 1,4-dienes. (a) Wang, C.-G.; Zhang, Y.; Wang, S.; Chen, B.; Li, Y.; Ni, H.-L.; Gao, Y.; Hu, P.; Wang, B.-Q.; Cao, P. Org. Lett. 2021, 23, 535. |
| [15] | (b) Li, Y.-Q.; Chen, G.; Shi, S.-L. Org. Lett. 2021, DOI: 10.1021/acs.orglett.1c00488. |
| [15] | (c) Li, Y.-Q.; Shi, S.-L. Organometallics 2021, DOI: 10.1021/acs.organomet.1c00096. |
/
| 〈 |
|
〉 |