

Chinese Journal of Organic Chemistry >
One-Pot Three-Component Synthesis of Novel 1,5-Benzodiazepine Derivatives and Their anti-BVDV (Bovine Viral Diarrhea Virus) Activity
Received date: 2020-04-12
Revised date: 2020-06-17
Online published: 2020-09-29
Supported by
the Natural Science Foundation of Hebei Province(B2017209119); the Education Department of Hebei Province(Z2017013)
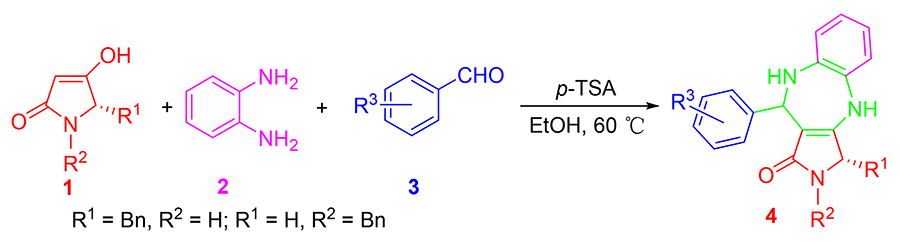
The “one-pot” three-component reaction is established to synthesize 1,5-benzodiazepine derivatives containing pyrrolidinone. These reactions were carried out readily with p-toluenesulfonic acid (p-TSA) as the catalyst in good yields. Reaction conditions were mild, and environmental friendly. The benzodiazepine derivatives displayed prominent anti-BVDV (bovine viral diarrhea virus) activity with excellent EC50 values and no significant cytotoxicity. Our research could afford significative compounds for the research of anti-BVDV agents.
Chao Han , Lei Nie , Xiao Han , Yan Zhang , Kelei Sun , Lei Shi , Guanghua Cui , Wei Meng . One-Pot Three-Component Synthesis of Novel 1,5-Benzodiazepine Derivatives and Their anti-BVDV (Bovine Viral Diarrhea Virus) Activity[J]. Chinese Journal of Organic Chemistry, 2021 , 41(2) : 819 -825 . DOI: 10.6023/202004018
| [1] | Han C.; Guo Y.-C.; Wang D.-D.; Dai X.-J.; Wu F.-J.; Liu H.-F.; Dai G.-F.; Tao J.-C. Chin. Chem. Lett. 2015, 26, 534. |
| [2] | Freedman H.; Logan M.R.; Law J. K. M.; Houghton M.ACS Infect. Dis. 2016, 2, 749. |
| [3] | Wetzel D.; Barbian A.; Jenzelewski V.; Schembecker G.; Merz J.; Michael Piontek, M.J. Biotechnol. 2019, 306, 203. |
| [4] | Darweesh M.F.; Rajput M. K. S.; Braun L.J.; Rohila J.S.; Chase C. C. L.Microb. Pathog. 2018, 121, 341. |
| [5] | (a) Buckwold V.E.; Wei J.; Wenzel-Mathers M.; Russell J. Antimicrob. Agents Chemother. 2003, 47, 2293. |
| [5] | (b) Yanagida K.; Baba C.; Baba M. Antiviral Res. 2004, 64, 195. |
| [6] | Wang L.-Z.; Hua Z.-X.; Niu W.-G. Chin. J. Org. Chem. 2010, 30, 1664. (in Chinese) |
| [6] | 王兰芝, 花中霞, 牛文刚, 有机化学, 2010, 30, 1664.). |
| [7] | Yin L.-Y.; Wang L.-Z.; Li X.-Q.; An Y.-S. Chin. J. Org. Chem. 2016, 36, 711. (in Chinese) |
| [7] | 尹刘燕, 王兰芝, 李晓庆, 安迎双, 有机化学, 2016, 36, 711.). |
| [8] | Thurston D.E.; Bose D.S.; Thompson A.S.; Howard P.W.; Leoni A.: Croker, S. J.; Jenkins, T.C.; Neidle, S.; Hartley, J.A.; Hurley, L.H.J. Org. Chem. 1996, 61, 8141. |
| [9] | Jaafar Z.; Chniti S.; Sassi A.B.; Dziri H.; Marque S.; Lecouvey M.; Gharbi R.; Msaddek M. J. Mol. Struct. 2019, 1195, 689. |
| [10] | (a) Drummer O.H. Forensic Sci. Rev. 2002, 14, 1. |
| [10] | (b) Hadjipavlou-Litina D.; Garg R.; Hansch C. Chem. Rev. 2004, 104, 3751. |
| [11] | Grossi G.; Braccio M. D.; Roma, G.; Ballabeni, V.; Tognolini, M.; Calcina, F.; Barocelli, E.Eur. J. Med. Chem. 2002, 37, 933. |
| [12] | Adegoke O.A.; Thomas O.E.; Makanjuola D.M.; Adewole O.O. J. Taibah Univ. Sci. 2014, 8, 248. |
| [13] | Kruse H. Drug Dev. Res. 1982, 2, 145. |
| [14] | Nicholson A.N.; Stone B.M.; Clarke C. H. J.Clin. Pharmacol. 1977, 4, 567. |
| [15] | Vendeville S.; Vandyck K.; Broeck W.V.; Boutton C.W.; Bondt H.D.; Quirynen L.; Amssoms K.; Bonfanti J.F.; Last S.; Rombauts K.; Tahri A.; Hu L.L.; Delouvroy F.; Vermeiren K.; Vandercruyssen G.; Helm L.V.; Cleiren E.; Mostmans W.; Lory P.; Pille G.; Emelen K.V.; Fanning G.; Pauwels F.; Lin T.; Simmen K.; Raboisson P. Bioorg. Med. Chem. Lett. 2009, 19, 2492. |
| [16] | Nyanguile O.; Pauwels F.; Van den Broeck W.; Boutton C.W.; Quirynen L.; Ivens T.; Van der Helm L.; Vandercruyssen G.; Mostmans W.; Delouvroy F.; Dehertogh P.; Cummings M.D.; Bonfanti J.-F.; Simmen K.A.; Raboisson P. Antimicrob. Agents Chemother. 2008, 52, 4420. |
| [17] | Lu L.-Q.; Chen J.-R.; Xiao W.-J. Acc. Chem. Res. 2012, 45, 1278. |
| [18] | Li X.-M.; Jia X.-S.; Yin L. Chin. J. Org. Chem. 2017, 37, 2237. (in Chinese) |
| [18] | 李修明, 贾学顺, 殷亮, 有机化学, 2017, 37, 2237.). |
| [19] | Liu B.-Y.; Xu X.-J.; Huang L.-L.; Feng H.-D. Chin. J. Org. Chem. 2020, 40, 1. (in Chinese) |
| [19] | 刘博瑜, 徐仙君, 黄立梁, 冯煌迪, 有机化学, 2020, 40, 1.). |
| [20] | Zhang M.; Liu Y.-H.; Shang Z.-R.; Hu H.-C.; Zhang Z.-H. Catal. Commun. 2017, 88, 39. |
| [21] | Deng J.; Mo L.-P.; Zhao F.-Y.; Hou L.-L.; Yang L.; Zhang Z.-H. Green Chem. 2011, 13, 2576. |
| [22] | Toure B.B.; Hall D.G. Chem. Rev. 2009, 109, 4439. |
| [23] | Ramon D.J.; Yus M. Angew. Chem., Int. Ed. 2005, 44, 1602. |
| [24] | Royles B. J. L.Chem. Rev. 1995, 95, 1981;. |
| [25] | Schobert R.; Schlenk A. Bioorg. Med. Chem. 2008, 16, 4203;. |
| [26] | Jeong Y.C.; Moloney M.G. Beilstein J. Org. Chem. 2013, 9, 1899. |
| [27] | (a) Ishida T.; Kobayashi R.; Yamada T. Org. Lett. 2014, 16, 2430;. |
| [27] | (b) Huang P.Q.; W.; Ye, J.L.Chin. J. Chem. 2015, 33, 655. |
| [28] | Sorokina I.K.; Alekseeva L.M.; Pashin V.A.; Asnina V.V.; Yuzhakov S.D.; Parimbetova R.B.; Granik V.G. Pharm. Chem. J. 1991, 25, 768. |
/
| 〈 |
|
〉 |