

Chinese Journal of Organic Chemistry >
A Concise Biogenetically Inspired Formal Synthesis of Camptothecin
Received date: 2021-02-20
Revised date: 2021-03-04
Online published: 2021-03-25
Supported by
National Natural Science Foundation of China(21871190)
A concise biogenetically inspired formal synthesis of camptothecin without use of protecting groups has been developed starting from tryptamine and easily prepared ethyl glutaconaldehyde salt. The synthesis features the key Pictet-Spengler reaction, efficient intramolecular oxa-Diels-Alder reaction to construct heptacyclic monoterpenoid indole alkaloid intermediate, as well as the following Winterfeldt biomimetic oxidation to form quinolinone moiety from indole skeleton.
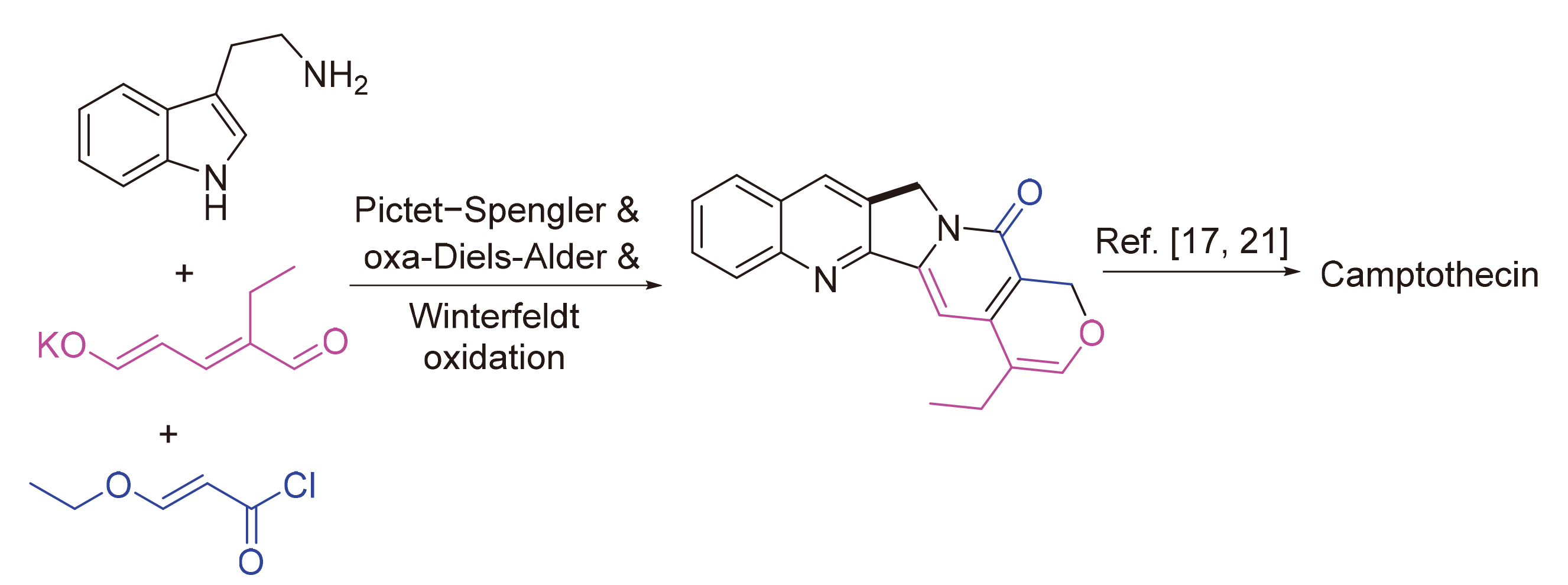
Yuanzhi Li , Mengqian Zhu , Liang Xu . A Concise Biogenetically Inspired Formal Synthesis of Camptothecin[J]. Chinese Journal of Organic Chemistry, 2021 , 41(7) : 2885 -2890 . DOI: 10.6023/cjoc202102034
| [1] | Wall,M. E.; Wani,M. C.; Cook,C. E.; Palmer,K. H.; McPhail,A. T.; Sim,G. A. J. Am. Chem. Soc. 1966, 88,3888. |
| [2] | Moertel,C. G.; Schutt,A. J.; Reitemeier,R. J.; Hahn,R. G. Cancer Chemother. Rep. 1972, 56,95. |
| [3] | (a) Hsiang,Y. H.; Hertzberg, R.; Hecht, S.; Liu,L. F. J. Biol. Chem. 1985, 260,14873. |
| [3] | (b) Hertzberg,R. P.; Busby,R. W.; Caranfa,M. J.; Holden,K. G.; John-son,R. K.; Hecht,S. M.; Kingsbury,W. D. J. Biol. Chem. 1990, 265,19287. |
| [3] | (c) Pommier, Y.; Kohlhagen, G.; Kohn,K. W.; Leteurtre, F.; Wani,M. C.; Wall,M. E. Proc. Natl. Acad. Sci. U. S. A. 1995, 92,8861. |
| [4] | (a) Uehling,D. E.; Nanthakumar,S. S.; Croom, D.; Emerson,D. L.; Leitner,P. P.; Luzzio,M. J.; McIntyre, G.; Morton, B.; Profeta, S. J. Med. Chem. 1995, 38,1106. |
| [4] | (b) Jew,S. S.; Kim,H. J.; Kim,M. G.; Roh,E. Y.; Cho,Y. S.; Kim,J. K.; Cha,K. H.; Lee,K. K.; Han,H. J.; Choi,J. Y.; Lee, H. Bioorg. Med. Chem. Lett. 1996, 6,845. |
| [4] | (c) Pan,X. D.; Han, R.; Sun,P. Y. Bioorg. Med. Chem. Lett. 2003, 13,3739. |
| [4] | (d) Tangirala,R. S.; Dixon, R.; Yang,D. Z.; Ambrus, A.; Antony, S.; Agama, K.; Pommier, Y.; Curran,D. P. Bioorg. Med. Chem. Lett. 2005, 15,4736. |
| [4] | (e) Hutchinson,C. R. Tetrahedron 1981, 37,1047. |
| [4] | (f) Du, W. Tetrahedron 2003, 59,8649. |
| [4] | (g) Thomas,C. J.; Rahier,N. J.; Hecht,S. M. Bioorg. Med. Chem. 2004, 12,1585. |
| [4] | (h) Li,Q. Y.; Zu,Y. G.; Shi,R. Z.; Yao,L. P. Curr. Med. Chem. 2006, 13,2021. |
| [4] | (i) Verma,R. P.; Hansch, C. Chem. Rev. 2009, 109,213. |
| [4] | (j) Martino, E.; Volpe,S. D.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. Bioorg. Med. Chem. Lett. 2017, 27,701. |
| [4] | (k) Pan, P.; Chen, J.; Li,M. Y.; Yu,H. D.; Zhao,J. J.; Ni, J.; Wang,X. W.; Sun,H. Y.; Tian, S.; Zhu, F.; Liu, F.; Huang, Y.; Hou,T. J. J. Med. Chem. 2018, 61,8613. |
| [5] | Kawato, Y.; Aonuma, M.; Hirota, Y.; Kuga, H.; Sato, K. Cancer Res. 1991, 51,4187. |
| [6] | Kingsbury,W. D.; Boehm,J. C.; Jakas,D. R.; Holden,K. G.; Hecht,S. M.; Gallagher, G.; Caranfa,M. J.; Mccabe,F. L.; Faucette,L. F.; Johnson,R. K.; Hertzberg,R. P. J. Med. Chem. 1991, 34,98. |
| [7] | Ban,H. J.; Oh,I. J.; Kim,K. S.; Ju,J. Y.; Kwon,Y. S.; Kim,Y. I.; Lim,S. C.; Kim,Y. C. Tuberc. Respir. Dis. 2009, 66,93. |
| [8] | Stork, G.; Schultz,A. G. J. Am. Chem. Soc. 1971, 93,4074. |
| [9] | For reviews, see: (a) Schultz,A. G. Chem. Rev. 1973, 73,385. |
| [9] | (b) Shamma, M.; Georgiev,V. St. J. Pharm. Sci. 1974, 63,163. |
| [9] | (c) Takayama, H.; Kitajima, M.; Aimi, N. J. Synth. Org. Chem. 1999, 57,181. |
| [9] | (d) Baurle, S.; Koert, U. In Organic Synthesis Highlights IV, Ed.: Schmalz, H. G., Wiley-VCH, Weinheim, 2000, p. 232. |
| [9] | (e) Kawato, Y.; Terasawa, H. Prog. Med. Chem. 1997, 34,69. |
| [9] | (f) Hutchinson,C. R. Tetrahedron 1981, 37,1047. |
| [9] | (g) Thomas,C. J.; Rahier,N. J.; Hecht,S. M. Bioorg. Med. Chem. 2004, 12,1585. |
| [9] | (h) Li,Q. Y.; Zu,Y. G.; Yao,L. P. Curr. Med. Chem. 2006, 13,2021. |
| [9] | (i) Liew,S. T.; Yang,L. X. Curr. Pharm. Des. 2008, 14,1078. |
| [9] | (j) Du, W. Tetrahedron 2003, 59,8649. |
| [9] | (k) Chen, L.; Chen,F. E. Synlett 2017, 28,1134. |
| [9] | For recent research articles after 2017, see: (l) Wang,X. L.; Xu,L. J.; Xiong,F. J.; Wu, Y.; Chen,F. E. Tetrahedron: Asymmetry 2017, 28,843. |
| [9] | (m) Yuan, Y.; Dong,W. H.; Gao,X. S.; Xie,X. M.; Curran,D. P.; Zhang,Z. G. Chin. J. Chem. 2018, 36,1035. |
| [9] | (n) Liu, Q.; Huang,G. X.; Liu,M. J.; Chen,F. E. Eur. J. Org. Chem. 2019,6024. |
| [9] | (o) Liu, Q.; Huang,G. X.; Liu,M. J.; Chen,F. E. Synthesis 2019, 51,3506. |
| [9] | (p) Liu, Q.; Liu,M. J.; Huang,G. X.; Chen,F. E. Tetrahedron 2019, 75,2647. |
| [9] | (q) Dong,W. H.; Yuan, Y.; Hu, B.; Gao,X. S.; Gao, H.; Xie,X. M.; Zhang,Z. G. Org. Lett. 2018, 20,80. |
| [10] | (a) Gaich, T.; Baran,P. S. J. Org. Chem. 2010, 75,4657. |
| [10] | (b) Zheng, K.; Shen,D. F.; Zhang,B. B.; Hong, R. J. Org. Chem. 2020, 85,13818. |
| [10] | (c) Vieira de Castro, T.; Yahiaoui, O.; Peralta, R.A.; Fallon, T.; Lee, V.; George, J.H. Org. Lett. 2020, 22,8161. |
| [11] | Wenkert, E.; Dave,K. G.; Lewis,R. G.; Sprague,P. W. J. Am. Chem. Soc. 1967, 89,6741. |
| [12] | (a) Winterfeldt, E.; Rodunz, H. Chem. Commun. 1971,374. |
| [12] | (b) Winterfeldt, E.; Korth, T.; Pike, D.; Boch, M. Angew. Chem.,Int. Ed. 1972, 11,289. |
| [13] | (a) Brown,R. T.; Leonard, J.; Sleigh,S. K. Phytochemistry 1978, 17,899. |
| [13] | (b) Brown,R. T.; Liu, J.; Santos,C. A.M., Tetrahedron Lett. 2000, 41,859. |
| [14] | Nguyen,T. M.; Peixoto, S.; Ouairy, C.; Nguyen,T. D.; Bénéchie, M.; Marazano, C.; Michel, P. Synthesis 2010,103. |
| [15] | Yan,L. H.; Skiredj, A.; Dory, Y.; Delpech, B.; Poupon, E. Eur. J. Org. Chem. 2014,4973. |
| [16] | (a) Nuhant, P.; Raikar,S. B.; Wypych,J. C.; Delpech, B.; Mazarano, C. J. Org. Chem. 2009, 74,9413. |
| [16] | (b) Overman,L. E.; Robichaud,A. J. J. Am. Chem. Soc. 1989, 111,300. |
| [17] | Liu,G. S.; Dong,Q. L.; Yao,Y. S.; Yao,Z. J. Org. Lett. 2008, 10,5393. |
| [18] | Yuan,Y. H.; Han, X.; Zhu,F. P.; Tian,J. M.; Zhang,F. M.; Zhang,X. M.; Tu,Y. Q.; Wang,S. H.; Guo, X. Nat. Commun. 2019, 10,3394. |
| [19] | Takayama, H.; Ishikawa, H.; Kurihara, M.; Kitajima, M.; Aimi, N.; Ponglux, D.; Koyama, F.; Matsumoto, K.; Moriyama, T.; Yamamoto,L. T.; Watanabe, K.; Murayama, T.; Horie, S. J. Med. Chem. 2002, 45,1949. |
| [20] | Thomas,O. P.; Zaparucha, A.; Husson,H. P. Eur. J. Org. Chem. 2002,157. |
| [21] | Li, K.; Ou,J. J.; Gao,S. H. Angew. Chem., nt. Ed. 2016, 55,14778. |
/
| 〈 |
|
〉 |