

Chinese Journal of Organic Chemistry >
Photosensitizer-Free Visible-Light-Promoted Trifluoromethylation of Imidazo[1,2-a]pyridines
Received date: 2021-03-02
Revised date: 2021-03-25
Online published: 2021-04-12
Supported by
National Natural Science Foundation of China(21625206); Strategic Priority Research Program of the Chinese Academy of Sciences(XDB20000000); Natural Science Foundation of Shanghai(20ZR1471600); Science and Technology Commission of Shanghai Municipality(19DZ2271100)
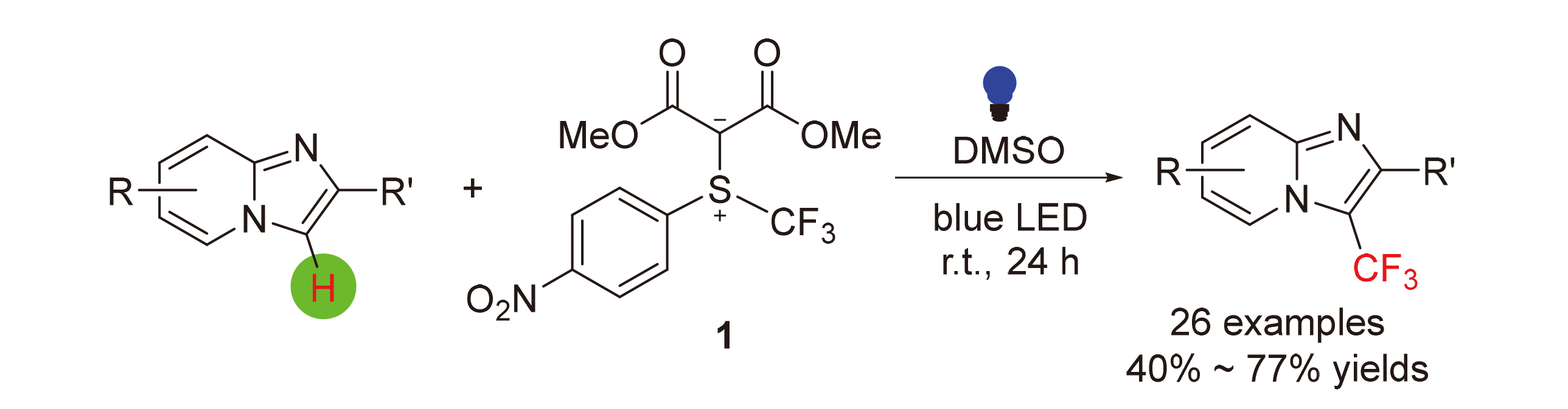
A photosensitizer-free visible-light-promoted method for direct trifluoromethylation of imidazo[1,2-a]pyridine derivatives using an electrophilic trifluoromethylating reagent based on sulfonium ylide skeleton was described. The reaction occurred in broad substrate scope under mild conditions and tolerant various functional groups. Initial mechanistic experiments including reactions in the presence of radical scavengers and UV absorption spectroscopic studies were conducted. Inhibition by the radical scavengers were observed. In addition, an extra-absorption peak between 500~510 nm in UV-Vis absorption spectrum was observed. These observations led us to propose a working mechanism. Finally, application of the current method for the preparation of trifluoromethylated derivative of gastroproctive drug zolimidine was demonstrated.
Ruichao Yao , Wenbo Chen , Qilong Shen . Photosensitizer-Free Visible-Light-Promoted Trifluoromethylation of Imidazo[1,2-a]pyridines[J]. Chinese Journal of Organic Chemistry, 2021 , 41(7) : 2684 -2692 . DOI: 10.6023/cjoc202103004
| [1] | (a) Krause, M.; Foks, H.; Gobis, K. Molecules 2017, 22, 399, doi: 10.3390/molecules22030399. |
| [1] | (b) Bagdi,A. K.; Santra, S.; Monir, K.; Hajra, A. Chem. Commun. 2015, 51,1555. |
| [2] | Zivkovic, B.; Morel, E.; Joly, D.; Perrault, G.; Sanger,D. J.; Lloyd,K. G. Pharmacopsychiatry 1990, 23,108. |
| [3] | Bomalaski,M. N.; Claflin,E. S.; Townsend, W.; Peterson,M. D. JAMA Neurol. 2017, 74,1130. |
| [4] | Materia, A.; Basso, N.; Bagarani, M.; Basoli, A.; Speranza, V. Clin. Ter. 1981, 97,183. |
| [5] | Sanger,D. J. Behav. Pharmacol. 1995, 6,116. |
| [6] | (a) Hagmann,W. K. J. Med. Chem. 2008, 51,4359. |
| [6] | (b) Kirk,K. L. Org. Process Res. Dev. 2008, 12,305. |
| [6] | (c) Meanwell,N. A. J. Med. Chem. 2011, 54,2529. |
| [6] | (d) Wang, J.; Sa?nchez-Rosello?, M.; Acen?a, J.; Pozo, C.; Sorochinsky,A. E.; Fustero, S.; Soloshonok,V. A.; Liu, H. Chem. Rev. 2014, 114,2432. |
| [7] | Monir, K.; Bagdi,A. K.; Ghosh, M.; Hajra, A. J. Org. Chem. 2015, 80,1332. |
| [8] | Wu, Y.; Zhang,H. -R.; Jin,R. -X.; Lan, Q.; Wang,X. -S. Adv. Synth. Catal. 2016, 358,3528. |
| [9] | Wang,R. -N.; Wang,J. -C.; Tang,Q. -X.; Zhao, X.; Wang,J. -F.; Leng,Y. -T.; Wu,Y. -J.; Chang,J. -B.; Wu,Y. -S.; Zhang,Z. -D.; Wang,S. -W. Tetrahedron Lett. 2019, 60,586. |
| [10] | Ji,X. -M.; We, L.; Chen, F.; Tang,R. -Y. RSC Adv. 2015, 5,29766. |
| [11] | Chen,X. -Y.; Ding,L. -C.; Li,L. -L.; Li,J. -Y.; Zou,D. -P.; Wu,Y. -J.; Wu,Y. -S. Tetrahedron Lett. 2020, 61,151538. |
| [12] | Han,S. -J.; Gao,X. -Y.; Wu,Q. -S.; Li,J. -Y.; Zou,D. -P.; Wu,Y. -J.; Wu,Y. -J. Adv. Synth. Catal. 2016, 3361,1559. |
| [13] | Lefebvre, Q.; Hoffmann, N.; Rueping, M. Chem. Commun. 2016, 52,2493. |
| [14] | Zhou,Q. -G.; Xu, S.; Zhang,R. -H. Tetrahedron Lett. 2019, 60,734. |
| [15] | Mi, X.; Kong,Y. -F.; Yang,H. -X.; Zhang,J. -Y.; Pi, C.; Cui,X. -L. Eur. J. Org. Chem. 2020,1019. |
| [16] | Liu,Y. -F.; Ling,Y. -J.; Lu, L.; Shen, Q. Chin. J. Chem. 2021, 39,1667. |
| [17] | Electrophilic fluoroalkylating reagents based on sulfonium or selenium-ylide skeleton, see: (a) Liu,Y. -F.; Shao,X. -X.; Lu, L; Shen, Q. Org. Lett. 2015, 17,2752. |
| [17] | (b) Zhu,J. -S.; Liu,Y. -F.; Shen, Q. Angew. Chem.,Int. Ed. 2016, 55,9050. |
| [17] | (c) Liu,Y. -F.; Lu, L.; Shen, Q. Angew. Chem.,Int. Ed. 2017, 56,9930. |
| [17] | (d) Zhu,J. -S.; Zheng,H. -L.; Xue,X. -S.; Xiao,Y. -S.; Liu,Y. -F.; Shen, Q. Chin. J. Chem. 2018, 36,1069. |
| [17] | (e) Ge,H. -M.; Shen, Q. Org. Chem. Front. 2019, 6,2205. |
| [17] | (f) Liu,Y. -F.; Ge,H. -M.; Lu, L.; Shen, Q. Chin. J. Org. Chem. 2019, 39,257 (in Chinese). |
| [17] | ( 刘亚飞, 葛航铭, 吕龙, 沈其龙, 有机化学, 2019, 39,257.) |
| [17] | (g) Hong, X.; Liu,Y. -F.; Lu, L.; Shen, Q. Chin. J. Chem. 2020, 38,1317. |
| [18] | Selected examples for phosphonium or sulfonium ylides in photoredox-catalyzed reactions: (a) Lu,L. -Q.; Li,T. -R.; Wang, Q.; Xiao,,W. -J. Chem. Soc. Rev. 2017, 46,4135. |
| [18] | (b) Das, M.; Vu,M. D.; Zhang, Q.; Liu,X. -W. Chem. Sci. 2019, 10,1687. |
| [18] | (c) Vu,M. D.; Leng,W. -L.; Hsu,H. -C.; Liu,X. -W. Asian J. Org. Chem. 2019, 8,93. |
| [18] | (d) Xia,X. -D.; Lu,L. -Q.; Liu,W. -Q.; Chen,D. -Z.; Zheng,Y. -H.; Wu,L. -Z.; Xiao,W. -J. Chem.-Eur. J. 2016, 22,8432. |
| [18] | (e) Yuan, F.; Yan,D. -M.; Gao,P. -P.; Shi,D. -Q.; Xiao,W. -J.; Chen,J. -R. ChemCatChem 2021, 12,543. |
| [19] | Related work for photo-trifluoromethylation via a donor-acceptor adduct, see: (a) Cheng,Y. -Z.; Yuan,X. -G.; Ma, J.; Yu,,S. -Y. Chem.- Eur. J. 2015, 21,8355. |
| [19] | (b) Macé, Y.; Pradet, C.; Popkin, M.; Blazejewski,J. -C.; Magnier, E. Tetrahedron Lett. 2010, 51,5388. |
| [20] | Pericherla, K.; Kaswan, P.; Khedara, P.; Khungara, B.; Parang, K.; Kumara, A. RSC Adv. 2013, 3,18923. |
/
| 〈 |
|
〉 |