

Chinese Journal of Organic Chemistry >
4-Dimethylaminopyridine-Boryl Radical Promoted Regioselective Radical Hydroboration of Electron-Deficient Alkenes
Received date: 2021-03-23
Revised date: 2021-04-09
Online published: 2021-04-25
Supported by
National Natural Science Foundation of China(21971226); Fundamental Research Funds for the Central Universities(WK2060000017)
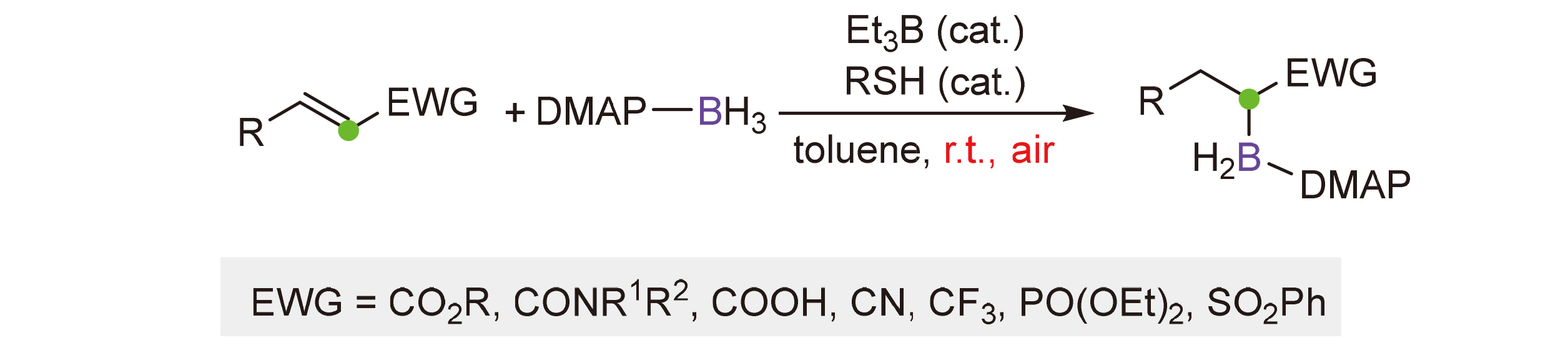
Organoboron compounds have shown significant applications in modern chemical synthesis. Hydroboration of alkenes is among the most widely used methods to access these targets. Herein, a regioselective radical hydroboration reaction of electron-deficient alkenes with 4-dimethylaminopyridine (DMAP)-boryl radical for the synthesis of α-boryl functionalized molecules is reported. The reaction features specific α-regioselectivity, mild reaction conditions, good functional group tolerance, and broad substrate scope. α,β-Unsaturated esters, amides, carboxylic acid, nitrile, trifluoromethyl molecule, sulfone, and phosphonate are viable substrates for this reaction. The resulting α-boryl functionalized molecules can be further transformed to various useful building blocks.
Yunshuai Huang , Xiaohui Jin , Fenglian Zhang , Yifeng Wang . 4-Dimethylaminopyridine-Boryl Radical Promoted Regioselective Radical Hydroboration of Electron-Deficient Alkenes[J]. Chinese Journal of Organic Chemistry, 2021 , 41(5) : 1957 -1967 . DOI: 10.6023/cjoc202103041
| [1] | Hall, D. G. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, 2nd ed., Wiley-VCH, Weinheim, Germany, 2011. |
| [2] | (a) Lei, Z.; Huang, Z. Synlett 2013, 24, 1745. |
| [2] | (b) Beletskaya, I.; Pelter, A. Tetrahedron 1997, 53, 4957. |
| [2] | (c) Burgess, K.; Ohlmeyer, M. J. Chem. Rev. 1991, 91, 1179. |
| [2] | (d) Chen, J.-B.; Whiting, A. Synthesis 2018, 50, 3843. |
| [3] | (a) Schiffner, J. A.; Müther, K.; Oestreich, M. Angew. Chem., Int. Ed. 2010, 49, 1194. |
| [3] | (b) Mantilli, L.; Mazet, C. ChemCatChem 2010, 2, 501. |
| [3] | (c) Calow, A. D. J.; Whiting, A. Org. Biomol. Chem. 2012, 10, 5485. |
| [3] | (d) Stavber, G.; ?asar, Z. ChemCatChem 2014, 6, 2162. |
| [3] | (e) Lee, S.; Yun, J. In Synthesis and Application of Organoboron Compounds, Vol. 49, Eds.: Fernandez, E.; Whiting, A., 2015, pp.73~92. |
| [3] | (f) Collins, B. S. L.; Wilson, C. M.; Myers, E. L.; Aggarwal, V. K. Angew. Chem., Int. Ed. 2017, 56, 11700. |
| [3] | (g) Liu, Y.; Zhang, W. Chin. J. Org. Chem. 2016, 36, 2249. (in Chinese). |
| [3] | (刘媛媛, 张万斌, 有机化学, 2016, 36, 2249.) |
| [4] | Ibrahim, M. R.; Bühl, M.; Knab, R.; Schleyer, P. V. R. J. Comput. Chem. 1992, 13, 423. |
| [5] | (a) He, Z.; Zajdlik, A.; Yudin, A. K. Acc. Chem. Res. 2014, 47, 1029. |
| [5] | (b) He, Z.; Zajdlik, A.; Yudin, A. K. Dalton Trans. 2014, 43, 11434. |
| [6] | Sucrow, W.; Zu?hlke, L.; Slopianka, M.; Pickardt, J. Chem. Ber. 1977, 110, 2818. |
| [7] | Ng, E. W. H.; Low, K.-H.; Chiu, P. J. Am. Chem. Soc. 2018, 140, 3537. |
| [8] | Radcliffe, J. E.; Fasano, V.; Adams, R. W.; You, P.; Ingleson, M. J. Chem. Sci. 2019, 10, 1434. |
| [9] | (a) Jin, J.; Xia, H.; Zhang, F.; Wang, Y. Chin. J. Org. Chem. 2020, 40, 2185. (in Chinese). |
| [9] | (靳继康, 夏慧敏, 张凤莲, 汪义丰, 有机化学, 2020, 40, 2185.) |
| [9] | (b) Zhang, F.-L.; Wang, Y.-F. In Science of Synthesis : Advances in Organoboron Chemistry towards Organic Synthesis, Ed.: Fernández, E., Thieme, Stuttgart, 2019, pp.355~392. |
| [9] | (c) Taniguchi, T. Eur. J. Org. Chem. 2019, 2019, 6308. |
| [9] | (d) Yang, J.; Li, Z.; Zhu, S. Chin. J. Org. Chem. 2017, 37, 2481. (in Chinese). |
| [9] | (杨吉民, 李子奇, 朱守非, 有机化学, 2017, 37, 2481.) |
| [9] | (e) Xu, A.-Q.; Zhang, F.-L.; Ye, T.; Yu, Z.-X.; Wang, Y.-F. CCS Chem. 2019, 1, 504. |
| [9] | (f) Ueng, S.-H.; Makhlouf Brahmi, M.; Derat, é.; Fensterbank, L.; Lac?te, E.; Malacria, M.; Curran, D. P. J. Am. Chem. Soc. 2008, 130, 10082. |
| [10] | (a) Ren, S.-C.; Zhang, F.-L.; Qi, J.; Huang, Y.-S.; Xu, A.-Q.; Yan, H.-Y.; Wang, Y.-F. J. Am. Chem. Soc. 2017, 139, 6050. |
| [10] | (b) Watanabe, T.; Hirose, D.; Curran, D. P.; Taniguchi, T. Chem. Eur. J. 2017, 23, 5404. |
| [10] | (c) Zhou, N.; Yuan, X.-A.; Zhao, Y.; Xie, J.; Zhu, C. Angew. Chem., Int. Ed. 2018, 57, 3990. |
| [10] | (d) Dai, W.; Geib, S. J.; Curran, D. P. J. Am. Chem. Soc. 2019, 141, 12355. |
| [10] | (e) Qi, J.; Zhang, F.-L.; Jin, J.-K.; Zhao, Q.; Li, B.; Liu, L.-X.; Wang, Y.-F. Angew. Chem., Int. Ed. 2020, 59, 12876. |
| [10] | (f) Xia, P. J.; Song, D.; Ye, Z. P.; Hu, Y. Z.; Xiao, J. A.; Xiang, H. Y.; Chen, X. Q.; Yang, H. Angew. Chem., Int. Ed. 2020, 59, 6706. |
| [10] | (g) Zhu, C.; Dong, J.; Liu, X.; Gao, L.; Zhao, Y.; Xie, J.; Li, S.; Zhu, C. Angew. Chem., Int. Ed. 2020, 59, 12817. |
| [10] | (h) Xu, W.; Jiang, H.; Leng, J.; Ong, H. W.; Wu, J. Angew. Chem., Int. Ed. 2020, 59, 4009. |
| [10] | (i) Dai, W.; Geib, S. J.; Curran, D. P. J. Am. Chem. Soc. 2020, 142, 6261. |
| [10] | (j) Kawamoto, T.; Morioka, T.; Noguchi, K.; Curran, D. P.; Kamimura, A. Org. Lett. 2021, 23, 1825. |
| [10] | (k) Liu, Y.; Li, J.-L.; Liu, X.-G.; Wu, J.-Q.; Huang, Z.-S.; Li, Q.; Wang, H. Org. Lett. 2021, 23, 1891. |
| [10] | Xu, H.; Jiang, Z. Chin. J. Org. Chem. 2020, 40, 3483. (in Chinese). |
| [10] | (许荷欢, 江智勇, 有机化学, 2020, 40, 3483.) |
| [11] | (a) Ren, S.-C.; Zhang, F.-L.; Xu, A.-Q.; Yang, Y.; Zheng, M.; Zhou, X.; Fu, Y.; Wang, Y.-F. Nat. Commun. 2019, 10, 1934. |
| [11] | (b) Huang, Y.-S.; Wang, J.; Zheng, W.-X.; Zhang, F.-L.; Yu, Y.-J.; Zheng, M.; Zhou, X.; Wang, Y.-F. Chem. Commun. 2019, 55, 11904. |
| [12] | Brahmi, M. M.; Monot, J.; Desage-El Murr, M.; Curran, D. P.; Fensterbank, L.; Lac?te, E.; Malacria, M. J. Org. Chem. 2010, 75, 6983. |
| [13] | (a) Lalevée, J.; Blanchard, N.; Tehfe, M.-A.; Chany, A.-C.; Fouassier, J.-P. Chem.-Eur. J. 2010, 16, 12920. |
| [13] | (b) Lalevée, J.; Blanchard, N.; Chany, A.-C.; Tehfe, M.-A.; Allonas, X.; Fouassier, J.-P. J. Phys. Org. Chem. 2009, 22, 986. |
| [13] | (c) Jin, J.-K.; Zhang, F.-L.; Wang, Y.-F. Acta Chim. Sinica 2019, 77, 889. (in Chinese). |
| [13] | (靳继康, 张凤莲, 汪义丰, 化学学报, 2019, 77, 889.) |
| [14] | Yu, Y.-J.; Zhang, F.-L.; Peng, T.-Y.; Wang, C.-L.; Cheng, J.; Chen, C.; Houk, K. N.; Wang, Y.-F. Science 2021, 371, 1232. |
| [15] | (a) Roberts, B. P. Chem. Soc. Rev. 1999, 28, 25. |
| [15] | (b) Pan, X.; Lac?te, E.; Lalevée, J.; Curran, D. P. J. Am. Chem. Soc. 2012, 134, 5669. |
| [16] | Staubitz, A.; Robertson, A. P. M.; Sloan, M. E.; Manners, I. Chem. Rev. 2010, 110, 4023. |
| [17] | (a) Griller, D.; Ingold, K. U. Acc. Chem. Res. 1980, 13, 317. |
| [17] | (b) Newcomb, M. Tetrahedron 1993, 49, 1151. |
| [18] | Dénès, F.; Pichowicz, M.; Povie, G.; Renaud, P. Chem. Rev. 2014, 114, 2587. |
| [19] | (a) Bonet, A.; Odachowski, M.; Leonori, D.; Essafi, S.; Aggarwal, V. K. Nat. Chem. 2014, 6, 584. |
| [19] | (b) Llaveria, J.; Leonori, D.; Aggarwal, V. K. J. Am. Chem. Soc. 2015, 137, 10958. |
/
| 〈 |
|
〉 |