

Chinese Journal of Organic Chemistry >
Sc(OTf)3-Catalyzed 1,6-Conjugate Addition of Thiols to δ-CF3-δ-aryl-disubstituted para-Quinone Methides: Efficient Construction of Diarylmethane Thioethers
Received date: 2021-03-23
Revised date: 2021-04-19
Online published: 2021-05-25
Supported by
National Natural Science Foundation of China(21801093); National Natural Science Foundation of China(21977021); National Natural Science Foundation of China(81760626); China Postdoctoral Science Foundation(2019M662321)
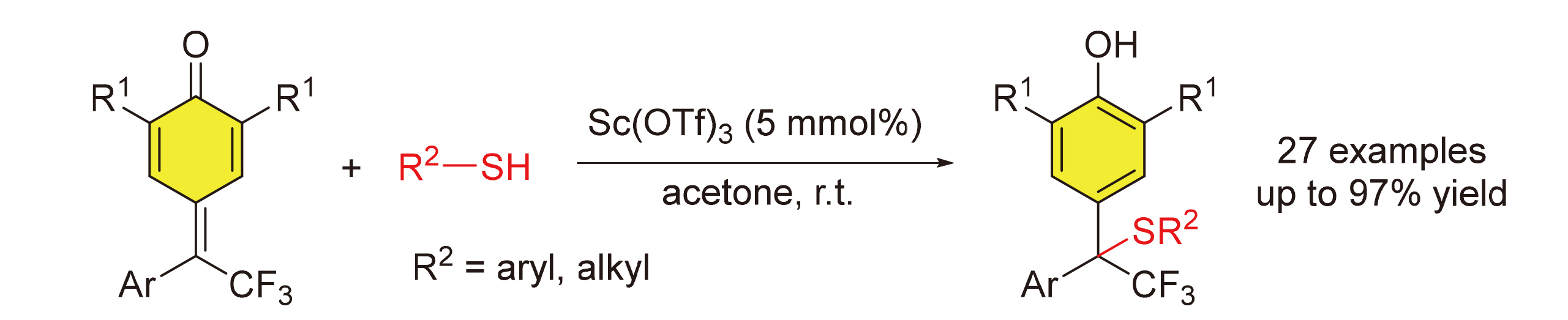
An efficient and practical 1,6-conjuate addition reaction of δ-CF3-δ-aryl-disubstituted para-quinone methides with thiols has been described. This approach provides a straightforward access to structurally diverse diarylmethane thioethers bearing CF3-substituted quaternary stereocenters using 5 mol% Sc(OTf)3 as catalyst. The reaction has an excellent functional-group tolerance, and displays a broad scope with respect to both δ-CF3-δ-aryl-disubstituted para-quinone methides and thiophenols. Moreover, alkyl thioalcohols and benzylmercaptan have proven to be suitable substrates. Diarylmethane thioethers belong to an important structural scaffold that widely exists in a number of bioactive molecules and trifluoromethyl group has a profound effect on physiological properties of organic molecules. Therefore, the efficient method for the synthesis of diarylmethane thioethers bearing CF3-substituted quaternary stereocenters might provide a powerful strategy for the discovery of biologically interesting agents.
Hua Li , Jingxiang Pang , Huazheng Liu , Changyin Zhao , Song Li , Hengshan Wang , Xigong Liu . Sc(OTf)3-Catalyzed 1,6-Conjugate Addition of Thiols to δ-CF3-δ-aryl-disubstituted para-Quinone Methides: Efficient Construction of Diarylmethane Thioethers[J]. Chinese Journal of Organic Chemistry, 2021 , 41(8) : 3134 -3143 . DOI: 10.6023/cjoc202103042
| [1] | (a) Kung, A. L.; Zabludoff, S. D.; France, D. S.; Freedman, S. J.; Tanner, E. A.; Vieira, A.; Cornell-Kennon, S.; Lee, J.; Wang, B.; Wang, J.; Memmert, K.; Naegeli, H.-U.; Petersen, F.; Eck, M. J.; Bair, K. W.; Wood, A. W.; Livingston, D. M. Cancer Cell 2004, 6, 33. |
| [1] | (b) Yurek-George, A.; Habens, F.; Brimmell, M.; Packham, G.; Ganesan, A. J. Am. Chem. Soc. 2004, 126, 1030. |
| [1] | (c) Jayakanthan, K.; Mohan, S.; Pinto, B. M. J. Am. Chem. Soc. 2009, 131, 5621. |
| [1] | (d) Krutetskaya, Z. I.; Milenina, L. S.; Naumova, A. A.; Antonov, V. G.; Nozdrachev, A. D. Dokl. Biochem. Biophys. 2016, 469, 302. |
| [1] | (e) Muchmore, D. B. Oncologist 2000, 5, 388. |
| [2] | (a) McCooey, S. H.; Connon, S. J. Angew. Chem. Int. Ed. 2005, 44, 6367. |
| [2] | (b) Okino, T.; Hoashi, Y.; Takemoto, Y. J. Am. Chem. Soc. 2003, 125, 12672. |
| [2] | (c) Stang, E. M.; Christina White, M. Nat. Chem. 2009, 1, 547. |
| [2] | (d) Lai, H.; Huang, Z.; Wu, Q.; Qin, Y. J. Org. Chem. 2009, 74, 283. |
| [2] | (e) Ji, Y.; Riera, A.; Verdaguer, X. Org. Lett. 2009, 11, 4346. |
| [2] | (f) Fang, G. Y.; Wallner, O. A.; Di Blasio, N.; Ginesta, X. Harvey, J. N.; Aggarwal, V. K. J. Am. Chem. Soc. 2007, 129, 14632. |
| [2] | (g) Unthank, M. G.; Tavassoli, B.; Aggarwal, V. K. Org. Lett. 2008, 10, 1501. |
| [3] | (a) Devendar, P.; Yang, G.-F. Top. Curr. Chem. 2017, 375, 82. |
| [3] | (b) Dubbaka, S. R.; Vogel, P. Angew. Chem. Int. Ed. 2005, 44, 7674. |
| [3] | (c) Cohen, A.; Crozet, M. D.; Rathelot, P.; Azas, N.; Vanelle, P. Molecules 2013, 18, 97. |
| [4] | (a) Kondo, T.; Mitsudo, T.-A. Chem. Rev. 2000, 100, 3205. |
| [4] | (b) Lee, C.-F.; Liu, Y.-C.; Badsara, S. S. Chem.-Asian J. 2014, 9, 706. |
| [4] | (c) Saidhareddy, N. Wang, P.; Jiang, X. Nat. Prod. Rep. 2020, 37, 246. |
| [4] | (d) Hosseinian, A.; Arshadi, S.; Sarhandi, S.; Monfared, A.; Vessally, E. J. Sulfur Chem. 2019, 40, 289. |
| [4] | (e) Li, J.; Yang, S.; Wu, W. Jiang, H. Org. Chem. Front. 2020, 7, 1395. |
| [4] | (f) Emmett, E. J.; Willis, M. C. Asian J. Org. Chem. 2015, 4, 602. |
| [5] | (a) Kao, R. Y. T.; Jenkins, J. L.; Olson, K. A.; Key, M. E.; Fett, J. W.; Shapiro, R. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 10066. |
| [5] | (b) Goyal, M.; Singh, P.; Alam, A.; Das, S. K.; Iqbal, M. S.; Dey, S.; Bindu, S.; Pal, C.; Das, S. K.; Panda, G.; Bandyopadhyay, U. Free Radical Biol. Med. 2012, 53, 129. |
| [5] | (d) Kumar, R. Drugs 2008, 68, 1803. |
| [5] | (e) Gouliaev, A. H.; Slok, F. A.; Teuber, L.; Demnitz, J. US 7429618, 2008. |
| [5] | (f) Langler, R. F.; Paddock, R. L.; Thompson, D. B.; Crandall, I.; Ciach, M.; Kain, K. C. Aust. J. Chem. 2003, 56, 1127. |
| [5] | (g) Ito, N.; Kurimura, M.; Yamauchi, T.; Segawa, C.; Sasaki, H.; Tai, K.; Arai, K.; Shinohara, T. WO 2009145357, 2009. |
| [6] | (a) Turner, A. B. Q. Rev. Chem. Soc. 1964, 18, 347. |
| [6] | (b) Peter, M. G. Angew. Chem. Int. Ed. 1989, 28, 555. |
| [6] | (c) Huang, X. Y.; Ding, R.; Mo, Z. Y.; Xu, Y. L.; Tang, H. T.; Wang, H. S.; Chen, Y. Y.; Pan, Y. M. Org. Lett. 2018, 20, 4819. |
| [6] | (d) Wu, Q. Y.; Ao, G. Z.; Liu, F. Org. Chem. Front. 2018, 5, 2061. |
| [6] | (e) Ke, M.; Song, Q. Adv. Synth. Catal. 2017, 359, 384. |
| [6] | (f) Li, S.; Liu, Y.; Huang, B.; Zhou, T.; Tao, H.; Xiao, Y.; Liu, L.; Zhang, J. ACS Catal. 2017, 7, 2805. |
| [6] | (g) Huang, G. B.; Huang, W. H.; Guo, J.; Xu, D. L.; Qu, X. C.; Zhai, P. H.; Zheng, X. H.; Weng, J.; Lu, G. Adv. Synth. Catal. 2019, 361, 1241. |
| [6] | (h) Chu, W. D.; Zhang, L. F.; Bao, X.; Zhao, X. H.; Zeng, C.; Du, J. Y.; Zhang, G. B.; Wang, F. X.; Ma, X. Y.; Fan, C. A. Angew. Chem. Int. Ed. 2013, 52, 9229. |
| [6] | (i) Lou, Y.; Cao, P.; Jia, T.; Zhang, Y.; Wang, M.; Liao, J. Angew. Chem. Int. Ed. 2015, 54, 12134. |
| [6] | (j) Wang, J.-Y.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Org. Chem. Front. 2020, 7, 1743. |
| [6] | (k) Zuo, H.-D.; Hao, W.-J.; Zhu, C.-F.; Guo, C.; Tu, S.-J.; Jiang, B. Org. Lett. 2020, 22, 4471. |
| [6] | (l) Chen, K.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Green Chem. 2019, 21, 675. |
| [7] | (a) Alejandro, P.; Mariola, T. ChemCatChem 2015, 7, 1524. |
| [7] | (b) Gai, K.; Fang, X.; Li, X.; Xu, J.; Wu, X.; Lin, A.; Yao, H. Chem. Commun. 2015, 51, 15831. |
| [7] | (c) Goswami, P.; Singh, G.; Anand, R. V. Org. Lett. 2017, 19, 1982. |
| [7] | (d) Liao, J. Y.; Ni, Q.; Zhao, Y. Org. Lett. 2017, 19, 4074. |
| [7] | (e) Shirsath, S. R.; Shinde, G. H.; Shaikh, A. C.; Muthukrishnan, M. J. Org. Chem. 2018, 83, 12305. |
| [7] | (f) Roy, D.; Panda, G. Synthesis 2019, 51, 4434. |
| [7] | (g) Terashima, K.; Kawasaki-Takasuka, T.; Agou, T.; Kubota, T.; Yamazaki, T. Chem. Commun. 2020, 56, 3031. |
| [7] | (h) Xiu, H.; Li, T.; Song, C.; Ma, Y. Eur. J. Org. Chem. 2020, 6068. |
| [7] | (i) Wang, Z.; Zhu, Y.; Pan, X.; Wang, G.; Liu, L. Angew. Chem. Int. Ed. 2020, 59, 3053. |
| [7] | (j) Pan, X.; Wang, X.; Kan, L.; Mao, Y.; Zhu, Y.; Liu, L. Chem. Sci. 2020, 11, 2414. |
| [7] | (k) Zhang, S.; Zhao, Y.; Li, Q.; Zhang, J.; Hou, Z.; Liu, Y.; Yu, Y.; Peng, D.; Wang, F.; Li, B.; Li, J. Chin. J. Org. Chem. 2019, 39, 709. (in Chinese) |
| [7] | (张硕, 赵宁, 李庆刚, 张嘉祺, 侯梓桐, 刘一帆, 于一涛, 彭丹, 王峰, 李冰, 李金辉, 有机化学, 2019, 39, 709.) |
| [7] | (l) Zhang, S.; Peng, D.; Zhao, Y.; Yu, Y.; Wang, F.; Liu, H.; Yi, G. Chin. J. Org. Chem. 2019, 39, 555. (in Chinese) |
| [7] | (张硕, 彭丹, 赵宁, 于一涛, 王峰, 刘海龙, 伊港, 有机化学, 2019, 39, 555.) |
| [8] | (m) Liu, L.; Zhang, J.-L. Chin. J. Org. Chem. 2019, 39, 3308. (in Chinese) |
| [8] | (刘路, 张俊良, 有机化学, 2019, 39, 3308.) |
| [9] | (a) Guan, X.-Y.; Zhang, L.-D.; You, P.-S.; Liu, S.-S.; Liu, Z.-Q. Tetrahedron Lett. 2019, 60, 244. |
| [9] | (b) Das, D.; Ghosh, K. G.; Chandu, P.; Sureshkumar, D. J. Org. Chem. 2020, 85, 14201. |
| [9] | (c) Liu, T.; Liu, J.; Xia, S.; Meng, J.; Shen, X.; Zhu, X.; Chen, W.; Sun, C.; Cheng, F. ACS Omega 2018, 3, 1409. |
| [9] | (d) Dong, N.; Zhang, Z.-P.; Xue, X.-S.; Li, Xin.; Cheng, J.-P. Angew. Chem. Int. Ed. 2016, 55, 1460. |
| [9] | (e) Jadhav, A. S.; Anand, R. V. Eur. J. Org. Chem. 2017, 3716. |
| [9] | (f) Liang, X.; Xu, H.; Li, H.; Chen, L.; Lu, H. Eur. J. Org. Chem. 2020, 217. |
| [9] | (g) Dubeya, A.; Manda, P. K. Synlett. 2020, 31, 1713. |
| [10] | (a) Ma, Y.; Pang, J.; Pan, X.; Ma, S.; Liu, X.; Liu, L. Synlett 2020, 31, 1619. |
| [10] | (b) Qi, Y.; Zhang, F.; Wang, L.; Feng, A.; Zhu, R.; Sun, S. Li, W.; Liu, L. Org. Biomol. Chem. 2020, 18, 3522. |
| [10] | (c) Pan, X; Cao, M.; Li, S.; Wang, H.; Liu, X.; Liu, L. Eur. J. Org. Chem. 2021, 1643. |
| [10] | (d) Wang, L.; Wang, N.; Qi, Y.; Liu, X.; Li, W.; Liu, L. Chin. J. Org. Chem. 2020, 40, 3934. (in Chinese) |
| [10] | (王琳, 王楠, 齐越, 孙书涛, 刘希功, 李伟, 刘磊, 有机化学, 2020, 40, 3934.) |
| [11] | (a) Petrov, V. A. Fluorinated Heterocyclic Compounds: Synthesis Chemistry, and Applications, Wiley, Hoboken, 2009. |
| [11] | (b) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455. |
| [11] | (c) Charpentier, J.; Früh, N.; Togni, A. Chem. Rev. 2015, 115, 650. |
| [11] | (d) Liu, X.; Xu, C.; Wang, M.; Liu, Q. Chem. Rev. 2015, 115, 683. |
| [11] | (e) Campbell, M. G.; Ritter, T. Chem. Rev. 2015, 115, 612. |
| [11] | (f) Ni, C.; Hu, M.; Hu, J. Chem. Rev. 2015, 115, 765. |
| [11] | (g) Wang, Z.; Mao, Y.; Guan, H.; Cao, M.; Hua, J.; Feng, L.; Liu, L. Chin. Chem. Lett. 2019, 30, 1241. |
| [11] | (h) Zhao, R.; Feng, G.; Xin, X.; Guan, H.; Hua, J.; Wan, R.; Li, W.; Liu, L. Chin. Chem. Lett. 2019, 30, 1432. |
| [12] | Jiang, C.; Chen, Y.; Huang, G.; Ni, C.; Liu, X.; Lu, H. Asian J. Org. Chem. 2019, 8, 257. |
/
| 〈 |
|
〉 |