

Chinese Journal of Organic Chemistry >
Reduction of Trifluoromethyl Ketones with Diethyl Zinc Catalyzed by Chiral Monophosphoryl Protected Diamine
Received date: 2021-02-26
Revised date: 2021-04-02
Online published: 2021-06-08
Supported by
Outstanding Teaching Team of 2019 “Blue Project” in Jiangsu Colleges and Universities(2019-69)
In order to obtain trifluoromethylated organic compounds with important biological activity, using phosphoramide as catalyst, diethylzinc and trifluoromethyl aromatic aldehyde as reactants, chiral trifluoromethylated organic compounds were synthesized through catalytic asymmetricβ-H transfer reduction reaction. The raw materials are cheap and easy to obtain, and the catalytic efficiency is high. The yield and ee value can be guaranteed to be highly and simultaneously under the optimized reaction conditions. Despite the large amount of catalyst, the ligand is very convenient to recycle and reuse in this system. At the same time, the reaction mechanism was speculated, and it was considered that the high stereoselectivity of the reaction was due to the formation of two six membered ring transition states and steric hindrance.
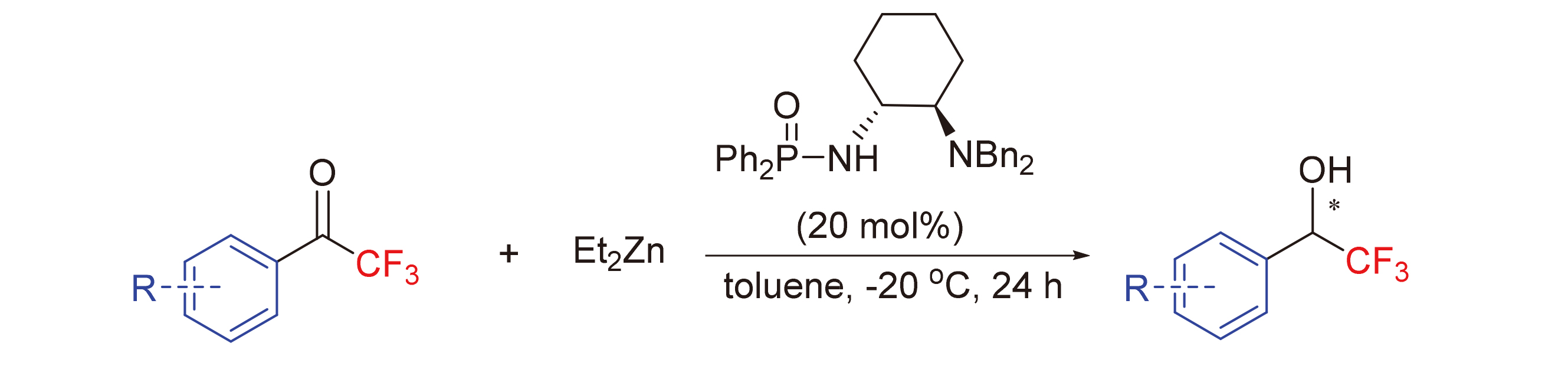
Xue Wang . Reduction of Trifluoromethyl Ketones with Diethyl Zinc Catalyzed by Chiral Monophosphoryl Protected Diamine[J]. Chinese Journal of Organic Chemistry, 2021 , 41(7) : 2693 -2699 . DOI: 10.6023/cjoc202102048
| [1] | (a) Xu,W. Y.; Feng,Y. S. Chem. J. Chin. Univ. 2020, 41,1567 (in Chinese). |
| [1] | ( 徐文艺, 冯乙巳, 高等学校化学学报, 2020, 41,1567.) |
| [1] | (b) Hu,Y. L.; Yang,T. Y.; Deng,Z. B.; Wang,K. H.; Li,P. F.; Huang,D. F.; Su,Y. P. J. Org. Chem. 2020, 85,12304. |
| [1] | (c) Xie,Q. Q.; Hu,J. B. Chin. J. Chem. 2020, 38,202. |
| [1] | (d) Schlosser, M. Angew. Chem.,Int. Ed. 2006, 45,5432. |
| [1] | (e) Muller, K.; Faeh, C.; Diederich, F. Science 2007, 317,1881. |
| [1] | (f) Purser, S.; Moore,P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37,320. |
| [1] | (g) Kirk,K. L. Org. Process Res. Dev. 2008, 12,305. |
| [2] | Shibatomi, K.; Narayama, A.; Abe Y.; Iwasa, S. Chem. Commun. 2012, 48,7380. |
| [3] | Endeshaw,M. M.; Li, C.; Leon,J. D.; Yao, N.; Latibeaudiere, K.; Premalatha, K.; Morrissette, N.; Werbovetz, K. Bioorg. Med. Chem. Lett. 2010, 20,5179. |
| [4] | Plosker,G. L.; Perry,C. M.; Goa,K. L. Pharmacoeconomics 2001, 19,421. |
| [5] | Ikeda, M.; Takahashi, K.; Dan, A.; Koyama, K.; Kubota, K.; Tanaka, T.; Hayashi, M. Bioorg. Med. Chem. 2000, 8,2157. |
| [6] | Bednarz,M. S.; Paul,S. D.; Kanamarlapudi,R. C.; Perlberg, A.; Zhang,H. M.. US 8653094 2014. |
| [7] | (a) Ohkuma, T.; Koizumi, M.; Doucet, H.; Pham, T.; Kozawa, M.; Murata, K.; Katayama, E.; Yokozawa, T.; Ikariya, T.; Noyori, R. J. Am. Chem. Soc. 1998, 120,13529. |
| [7] | (b) Kuroki, Y.; Asada, D.; Iseki, K. Tetrahedron Lett. 2000, 41,9853. |
| [7] | (c) Kuroki, Y.; Sakamaki, Y.; Iseki, K. Org. Lett. 2001, 3,457. |
| [7] | (d) Sterk, D.; Stephan, M.; Mohar,B. S.; Szollosi, G.; Bartok, M. Appl. Catal. A: Gen. 2009, 362,178. |
| [7] | (f) Pereniguez, R.; Santarossa, G.; Mallat, T.; Baiker, A. J. Mol. Catal. A: Chem. 2012, 365,39. |
| [8] | (a) Gaspar, J.; Guerrero, A. Tetrahedron: Asymmetry 1995, 6,231. |
| [8] | (b) Nakamura, K.; Matsuda, T.; Itoh, T.; Ohno, A. Tetrahedron Lett. 1996, 37,5727. |
| [8] | (c) Nakamura, K.; Matsuda, T.; Shimizu, M.; Fujisawa, T. Tetrahedron 1998, 54,8393. |
| [8] | (d) Inoue, K.; Makino, Y.; Itoh, N. Tetrahedron: Asymmetry 2005, 16,2539. |
| [8] | (e) Borzecka, W.; Lavandera, I.; Gotor, V. J. Org. Chem. 2013, 78,7312. |
| [9] | (a) Yamaguch, S.; Mosher,H. S. J. Org. Chem. 1973, 38,1870. |
| [9] | (b) Pirkle,W. H.; Sikkenga,D. L.; Pavlin,M. S. J. Org. Chem. 1977, 42,384. |
| [9] | (c) Hawkins,J. M.; Sharpless,K. B. J. Org. Chem. 1984, 49,3861. |
| [9] | (d) Chong,J. M.; Mar,E. K. J. Org. Chem. 1991, 56,893. |
| [10] | (a) Stepanenko, V.; De,J. M.; Correa, W.; Guzman, I.; Vazquez, C.; Cruz, W.; Ortiz-Marciales, M.; Barnes,C. L. Tetrahedron Lett. 2007, 48,5799. |
| [10] | (b) Korenaga, T.; Nomura, K.; Onoue, K.; Sakai, T. Chem. Commun. 2010, 46,8624. |
| [10] | (c) Kawanami, Y.; Hoshino, K.; Tsunoi, W. Tetrahedron: Asymmetry 2011, 22,1464. |
| [10] | (d) Turgut, Y.; Azizoglu, M.; Erdogan, A.; Arslan, N.; Hosgoren, H. Tetrahedron: Asymmetry 2013, 24,853. |
| [10] | (e) Harauchi, Y.; Takakura, C.; Furumoto, T.; Yanagita,R. C.; Kawanami, Y. Tetrahedron: Asymmetry 2015, 26,333. |
| [11] | (a) Nasipuri, D.; Bhattacharya,P. K. J. Chem. Soc.,Perkin Trans. 1 1977,576. |
| [11] | (b) Morrison,J. D.; Tomaszewski,J. E.; Mosher,H. S.; Dale, J.; Miller, D.; Elsenbaumer,R. L. J. Am. Chem. Soc. 1977, 99,3167. |
| [11] | (c) Yong,K. H.; Chong,J. M. Org. Lett. 2002, 4,4139. |
| [12] | Sasaki, S.; Yamauchi, T.; Kubo, H.; Kanai, M.; Ishii, A.; Higashiyama, K. Tetrahedron Lett. 2005, 46,1497. |
| [13] | (a) Yearick, K.; Wolf, C. Org. Lett. 2008, 10,3915. |
| [13] | (b) Genov, M.; Martinez-Ilarduya,J. M.; Calvillo-Barahona, M.; Espinet, P. Organometallic 2010, 29,6402. |
| [13] | (c) Calvillo-Barahona, M.; Cordovilla, C.; Genov,M. N.; Martinez-Ilarduya,J. M.; Espinet, P. Dalton Trans. 2013, 42,14576. |
| [13] | (d) Calvillo-Barahona, M.; Casares,J. A.; Cordovilla, C.; Genov,M. N.; Martinez-Ilarduya,J. M.; Espinet, P. Chem.-Eur. J. 2014, 20,14800. |
| [14] | Huang, H.; Zong, H.; Bian,G. L.; Song, L. Tetrahedron: Asymmetry 2015, 26,835. |
| [15] | (a) Forni, A.; Moretti, I.; Prati, F.; Torre, G. Tetrahedron 1994, 50,11995. |
| [15] | (b) Asao, N.; Asano, T.; Yamamoto, Y. Angew. Chem.,Int. Ed. 2001, 40,3206. |
| [15] | (c) Hess, R.; Diezi, S.; Mallat, T.; Baiker, A. Tetrahedron: Asymmetry 2004, 15,251. |
| [15] | (d) Diezi, S.; Hess, M.; Orglmeister, E.; Mallat, T.; Baiker, A. Catal. Lett. 2005, 102,121. |
| [15] | (e) Grau,B. T.; Devine,P. N.; DiMichele,L. N.; Kosjek, B. Org. Lett. 2007, 9,4951. |
| [16] | (a) Huang,H. Y.; Zong, H.; Bian,G. L.; Song, L. J. Org. Chem. 2012, 77,10427. |
| [16] | (b) Zong, H.; Huang,H. Y.; Bian,G. L.; Song, L. Tetrahedron Lett. 2013, 54,2722. |
| [16] | (c) Shen, B.; Huang,H. Y.; Bian,G. L.; Zong, H.; Song, L. Chirality 2013, 25,561. |
| [16] | (d) Huang,H. Y.; Zong, H.; Shen, B.; Yue,H. F.; Bian,G. L.; Song, L. Tetrahedron 2014, 70,1289. |
| [16] | (e) Yue,H. F.; Huang,H. Y.; Zong, H.; Bian,G. L.; Zong, H.; Li,F. L.; Song, L. Tetrahedron: Asymmetry 2014, 25,170. |
| [17] | Hatano, M.; Miyamoto, T.; Ishihara, K. Org. Lett. 2007, 9,4535. |
| [18] | Huang,H. Y.; Zong, H.; Bian,G. L.; Song, L. J. Org. Chem. 2015, 80,12614. |
| [19] | González-Martínez, D.; Gotor, V.; Gotor-Fernández, V. ChemCatChem 2019, 11,5800. |
| [20] | Brüning, F.; Nagae, H.; Käch, D.; Mashima,K. I.; Togni, A. Chem.-Eur. J. 2019, 25,10818. |
/
| 〈 |
|
〉 |