

Chinese Journal of Organic Chemistry >
Progress in the Synthesis of C(sp2)—C(sp3) Bond by Reductive Heck Reactions of Alkenes
Received date: 2021-05-10
Revised date: 2021-05-31
Online published: 2021-06-29
Supported by
National Natural Science Foundation of China(21807051); Natural Science Foundation of Jiangxi Province(20202BABL213006)
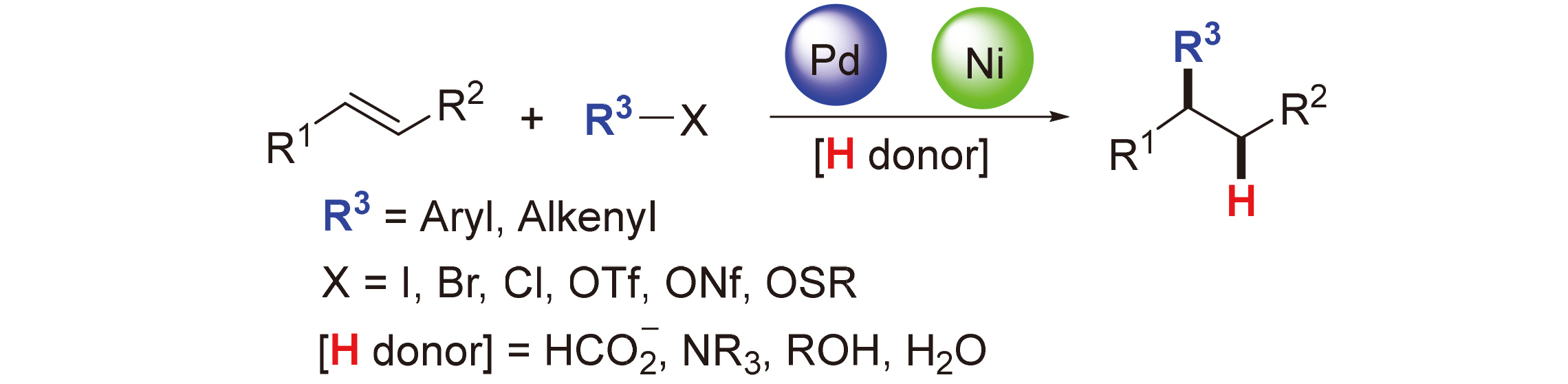
Transition-metal catalyzed C(sp2)—C(sp3) cross-coupling is of great significance in organic chemistry for synthesizing complex natural products and pharmaceutical molecules. Recently, reductive Heck reactions have emerged as one of the simple and efficient strategies for the construction of C(sp2)—C(sp3) bond. The recent advances in the reductive Heck reactions of alkenes are summarized on the basis of different hydride sources. The related mechanisms are provided, and the future development of this field is also prospected.
Xiao Xiao , Jianchao Liu . Progress in the Synthesis of C(sp2)—C(sp3) Bond by Reductive Heck Reactions of Alkenes[J]. Chinese Journal of Organic Chemistry, 2021 , 41(9) : 3349 -3365 . DOI: 10.6023/cjoc202105016
| [1] | Lovering, F.; Bikker, J.; Humblet, C. J. Med. Chem. 2009, 52, 6752. |
| [2] | (a) Rudolph, A.; Lautens, M. Angew. Chem., Int. Ed. 2009, 48, 2656. |
| [2] | (b) Jana, R.; Pathak, T. P.; Sigman, M. S. Chem. Rev. 2011, 111, 1417. |
| [2] | (c) Cherney, A. H.; Kadunce, N. T.; Reisman, S. E. Chem. Rev. 2015, 115, 9587. |
| [2] | (d) Dong, Z.; Ren, Z.; Thompson, S. J.; Xu, Y.; Dong, G. Chem. Rev. 2017, 117, 9333. |
| [2] | (e) Dong, X.; Hou, Y.; Meng, F.; Liu, H.; Liu, H. Chin. J. Org. Chem. 2017, 37, 1088. (in Chinese). |
| [2] | ( 董旭, 侯永正, 孟凡威, 刘洪波, 刘会, 有机化学, 2017, 37, 1088.) |
| [2] | (f) Dombrowski, A. W.; Gesmundo, N. J.; Aguirre, A. L.; Sarris, K. A.; Young, J. M.; Bogdan, A. R.; Martin, M. C.; Gedeon, S.; Wang, Y. ACS Med. Chem. Lett. 2020, 11, 597. |
| [3] | (a) McDonald, R. I.; Liu, G.; Stahl, S. S. Chem. Rev. 2011, 111, 2981. |
| [3] | (b) Coombs, J. R.; Morken, J. P. Angew. Chem., Int. Ed. 2016, 55, 2636. |
| [3] | (c) Yin, G.; Mu, X.; Liu, G. Acc. Chem. Res. 2016, 49, 2413. |
| [3] | (d) Zhang, J. S.; Liu, L.; Chen, T.; Han, L. B. Chem.-Asian J. 2018, 13, 2277. |
| [3] | (e) Li, Z.-L.; Fang, G.-C.; Gu, Q.-S.; Liu, X.-Y. Chem. Soc. Rev. 2020, 49, 32. |
| [3] | (f) Li, Y.; Wu, D.; Cheng, H.; Yin, G. Angew. Chem., Int. Ed. 2020, 59, 7990. |
| [4] | Cacchi, S.; Arcadi, A. J. Org. Chem. 1983, 48, 4236. |
| [5] | Diaz, P.; Gendre, F.; Stella, L.; Charpentier, B. Tetrahedron 1998, 54, 4579. |
| [6] | (a) Yang, X.; Ma, S.; Du, Y.; Tao, Y. Chin. J. Org. Chem. 2013, 33, 2325. (in Chinese). |
| [6] | ( 杨晓梅, 马莎, 杜艳妮, 陶云海, 有机化学, 2013, 33, 2325.) |
| [6] | (b) Diethelm, S.; Carreira, E. M. J. Am. Chem. Soc. 2013, 135, 8500. |
| [6] | (c) Oxtoby, L. J.; Gurak, J. A. Jr.; Wisniewski, S. R.; Eastgate, M. D.; Engle, K. M. Trends Chem. 2019, 1, 572. |
| [6] | (d) Ghosh, T. ChemistrySelect 2019, 4, 4747. |
| [6] | (e) Xie, J.-Q.; Liang, R.-X.; Jia, Y.-X. Chin. J. Chem. 2021, 39, 710. |
| [7] | (a) Heck, R. F.; Nolley, J. P. J. Org. Chem. 1972, 37, 2320. |
| [7] | (b) Cabri, W.; Candiani, I. Acc. Chem. Res. 1995, 28, 2. |
| [7] | (c) Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009. |
| [8] | Chen, J.-Q.; Xie, J.-H.; Bao, D.-H.; Liu, S.; Zhou, Q.-L. Org. Lett. 2012, 14, 2714. |
| [9] | Pu, L.-Y.; Chen, J.-Q.; Li, M.-L.; Li, Y.; Xie, J.-H.; Zhou, Q.-L. Adv. Synth. Catal. 2016, 358, 1229. |
| [10] | Peng, R.; Van Nieuwenhze, M. S. Org. Lett. 2012, 14, 1962. |
| [11] | Peng, R.; Van Nieuwenhze, M. S. J. Org. Chem. 2019, 84, 173. |
| [12] | (a) Ichikawa, M.; Takahashi, M.; Aoyagi, S.; Kibayashi, C. J. Am. Chem. Soc. 2004, 126, 16553. |
| [12] | (b) Okamura, H.; Mori, N.; Watanabe, H.; Takikawa, H. Tetrahedron Lett. 2018, 59, 4397. |
| [12] | (c) Zheng, Y.; Yue, B.-B.; Wei, K.; Yang, Y.-R. J. Org. Chem. 2018, 83, 4867. |
| [13] | Gao, P. G.; Cook, S. P. Org. Lett. 2012, 14, 3340. |
| [14] | Liu, S.; Zhou, J. Chem. Commun. 2013, 49, 11758. |
| [15] | (a) Arcadi, A.; Marinelli, F.; Bernocchi, E.; Cacchi, S.; Ortar, G. J. Organomet. Chem. 1989, 368, 249. |
| [15] | (b) Kasyan, A.; Wagner, C.; Maier, M. E. Tetrahedron 1998, 54, 8047. |
| [15] | (c) Namyslo, J. C.; Kaufmann, D. E. Synlett 1999, 6, 804. |
| [15] | (d) Carroll, F. I.; Liang, F.; Navarro, H. A.; Brieaddy, L. E.; Abraham, P.; Damaj, M. I.; Martin, B. R. J. Med. Chem. 2001, 44, 2229. |
| [15] | (e) Yao, M.-L.; Adiwidjaja, G.; Kaufmann, D. E. Angew. Chem., Int. Ed. 2002, 41, 3375. |
| [15] | (f) Namyslo, J. C.; Storsberg, J.; Klinge, J.; Gärtner, C.; Yao, M.-L.; Ocal, N.; Kaufmann, D. E. Molecules 2010, 15, 3402. |
| [15] | (g) Jana, G. K.; Sinha, S. Tetrahedron Lett. 2012, 53, 1671. |
| [15] | (h) Aida, F.; Sone, H.; Ogawa, R.; Hamaoka, T.; Shimizu, I. Chem. Lett. 2015, 44, 715. |
| [16] | Vijayan, A.; Jumaila, C. U.; Baiju, T. V.; Radhakrishnan, K. V. ChemistrySelect 2017, 2, 5913. |
| [17] | Shen, C.; Liu, R.-R.; Fan, R.-J.; Li, Y. -, L.; Xu, T.-F.; Gao, J.-R.; Jia, Y.-X. J. Am. Chem. Soc. 2015, 137, 4936. |
| [18] | Liu, R.-R.; Xu, Y.; Liang, R.-X.; Xiang, B.; Xie, H.-J.; Gao, J.-R.; Jia, Y.-X. Org. Biomol. Chem. 2017, 15, 5790. |
| [19] | Saini, V.; O'Dair, M.; Sigman, M. S. J. Am. Chem. Soc. 2015, 137, 608. |
| [20] | Dou, Y.; Yang, L.; Zhang, L.; Zhang, P.; Li, H.; Yang, G. RSC Adv. 2016, 6, 100632. |
| [21] | Hou, L.; Yuan, Y.; Tong, X. Org. Biomol. Chem. 2017, 15, 4803. |
| [22] | Liang, R.-X.; Yang, R.-Z.; Liu, R.-R.; Jia, Y.-X. Org. Chem. Front. 2018, 5, 1840. |
| [23] | Vargas, D. R.; Cook, S. P. Tetrahedron 2018, 74, 3314. |
| [24] | Gurak, J. A. Jr.; Engle, K. M. ACS Catal. 2018, 8, 8987. |
| [25] | Heck, R. F. Acc. Chem. Res. 1979, 12, 146. |
| [26] | Oxtoby, L. J.; Li, Z.-Q.; Tran, V. T.; Erbay, T. G.; Deng, R.; Liu, P.; Engle, K. M. Angew. Chem., Int. Ed. 2020, 59, 8885. |
| [27] | Zhang, Z.-M.; Xu, B.; Qian, Y.; Wu, L.; Wu, Y.; Zhou, L.; Liu, Y.; Zhang, J. Angew. Chem., Int. Ed. 2018, 57, 10373. |
| [28] | Diaz, P.; Phatak, S. S.; Xu, J.; Fronczek, F. R.; Astruc-Diaz, F.; Thompson, C. M.; Cavasotto, C. N.; Naguib, M. ChemMedChem 2009, 4, 1615. |
| [29] | Yuan, Z.; Feng, Z.; Zeng, Y.; Zhao, X.; Lin, A.; Yao, H. Angew. Chem., Int. Ed. 2019, 58, 2884. |
| [30] | Peng, X.; Yang, Y.; Luo, B.; Wen, S.; Huang, P. Adv. Synth. Catal. 2021, 363, 222. |
| [31] | Han, M.-L.; Huang, W.; Liu, Y.-W.; Liu, M.; Xu, H.; Xiong, H.; Dai, H.-X. Org. Lett. 2021, 23, 172. |
| [32] | Murahashi, S.-I.; Watanabe, T. J. Am. Chem. Soc. 1979, 101, 7429. |
| [33] | Minatti, A.; Zheng, X. L.; Buchwald, S. L. J. Org. Chem. 2007, 72, 9253. |
| [34] | Gottumukkala, A. L.; de Vries, J. G.; Minnaard, A. J. Chem.-Eur. J. 2011, 17, 3091. |
| [35] | Raoufmoghaddam, S.; Mannathan, S.; Minnaard, A. J.; de Vries, J. G.; Reek, J. N. H. Chem.-Eur. J. 2015, 21, 18811. |
| [36] | Mannathan, S.; Raoufmoghaddam, S.; Reek, J. N. H.; de Vries, J. G.; Minnaard, A. J. ChemCatChem 2015, 7, 3923. |
| [37] | Yue, G.; Lei, K.; Hirao, H.; Zhou, J. Angew. Chem., Int. Ed. 2015, 54, 6531. |
| [38] | Mannathan, S.; Raoufmoghaddam, S.; Reek, J. N. H.; de Vries, J. G.; Minnaard, A. J. ChemCatChem 2017, 9, 551. |
| [39] | Balanta, A.; Godard, C.; Claver, C. Chem. Soc. Rev. 2011, 40, 4973. |
| [40] | Parveen, N.; Saha, R.; Sekar, G. Adv. Synth. Catal. 2017, 359, 3741. |
| [41] | Parveen, N.; Sekar, G. Adv. Synth. Catal. 2019, 361, 4581. |
| [42] | (a) Broccatelli, F.; Cruciani, G.; Benet, L. Z.; Oprea, T. I. Mol. Pharmaceutics 2012, 9, 570. |
| [42] | (b) Cha, J.-Y.; Park, J.-M.; Lee, H.-J.; Bae, J.-S.; Han, Y.-M; Oh, B.-C.; Ko, K. H.; Hahm, K.-B. Curr. Pharm. Des. 2017, 23, 3941. |
| [43] | Wang, C.; Xiao, G.; Guo, T.; Ding, Y.; Wu, X.; Loh, T.-P. J. Am. Chem. Soc. 2018, 140, 9332. |
| [44] | (a) Zaitsev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127, 13154. |
| [44] | (b) Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2010, 132, 3965. |
| [44] | (c) Daugulis, O.; Roane, J.; Tran, L. D. Acc. Chem. Res. 2015, 48, 1053. |
| [45] | Ozeryanskii, V. A.; Gorbacheva, A. Y.; Pozharskii, A. F.; Vlasenko, M. P.; Tereznikov, A. Y.; Chernov'yants, M. S. Org. Biomol. Chem. 2015, 13, 8524 |
| [46] | Guo, T.; Ding, Y.; Zhou, L.; Xu, H.; Loh, T.-P.; Wu, X. ACS Catal. 2020, 10, 7262. |
| [47] | Harrington, P. J.; Lodewijk, E. Org. Process Res. Dev. 1997, 1, 72. |
| [48] | Raoufmoghaddam, S.; Mannathan, S.; Minnaard, A. J.; de Vries, J. G.; de Bruin, B.; Reek, J. N. H. ChemCatChem 2018, 10, 266. |
| [49] | Jin, L.; Qian, J.; Sun, N.; Hu, B.; Shen, Z.; Hu, X. Chem. Commun. 2018, 54, 5752. |
| [50] | Zheng, K.; Xiao, G.; Guo, T.; Ding, Y.; Wang, C.; Loh, T.-P.; Wu, X. Org. Lett. 2020, 22, 694. |
| [51] | (a) Rosen, B. M.; Quasdorf, K. W.; Wilson, D. A.; Zhang, N.; Resmerita, A. M.; Garg, N. K.; Percec, V. Chem. Rev. 2011, 111, 1346. |
| [51] | (b) Diccianni, J.; Lin, Q.; Diao, T. Acc. Chem. Res. 2020, 53, 906. |
| [51] | (c) Qi, X.; Diao, T. ACS Catal. 2020, 10, 8542. |
| [52] | Yang, F.; Jin, Y.; Wang, C. Org. Lett. 2019, 21, 6989. |
| [53] | Li, Y.; Gong, J.-F.; Song, M.-P. Org. Chem. Front. 2020, 7, 2216. |
| [54] | Yang, X.-W.; Li, D.-H.; Song, A.-X.; Liu, F.-S. J. Org. Chem. 2020, 85, 11750. |
| [55] | Kong, W.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2017, 56, 3987. |
| [56] | Qin, X. R.; Lee, M. W. Y.; Zhou, J. S. Angew. Chem., Int. Ed. 2017, 56, 12723. |
| [57] | Qin, X.; Lee, M. W. Y.; Zhou, J. S. Org. Lett. 2019, 21, 5990. |
| [58] | Huang, X.; Teng, S.; Chi, Y. R.; Xu, W.; Pu, M.; Wu, Y.-D.; Zhou, J. S. Angew. Chem., Int. Ed. 2021, 60, 2828. |
/
| 〈 |
|
〉 |