

Chinese Journal of Organic Chemistry >
Progress in the Synthesis of 3-Substituted Phthaides
Received date: 2021-04-30
Revised date: 2021-05-26
Online published: 2021-07-06
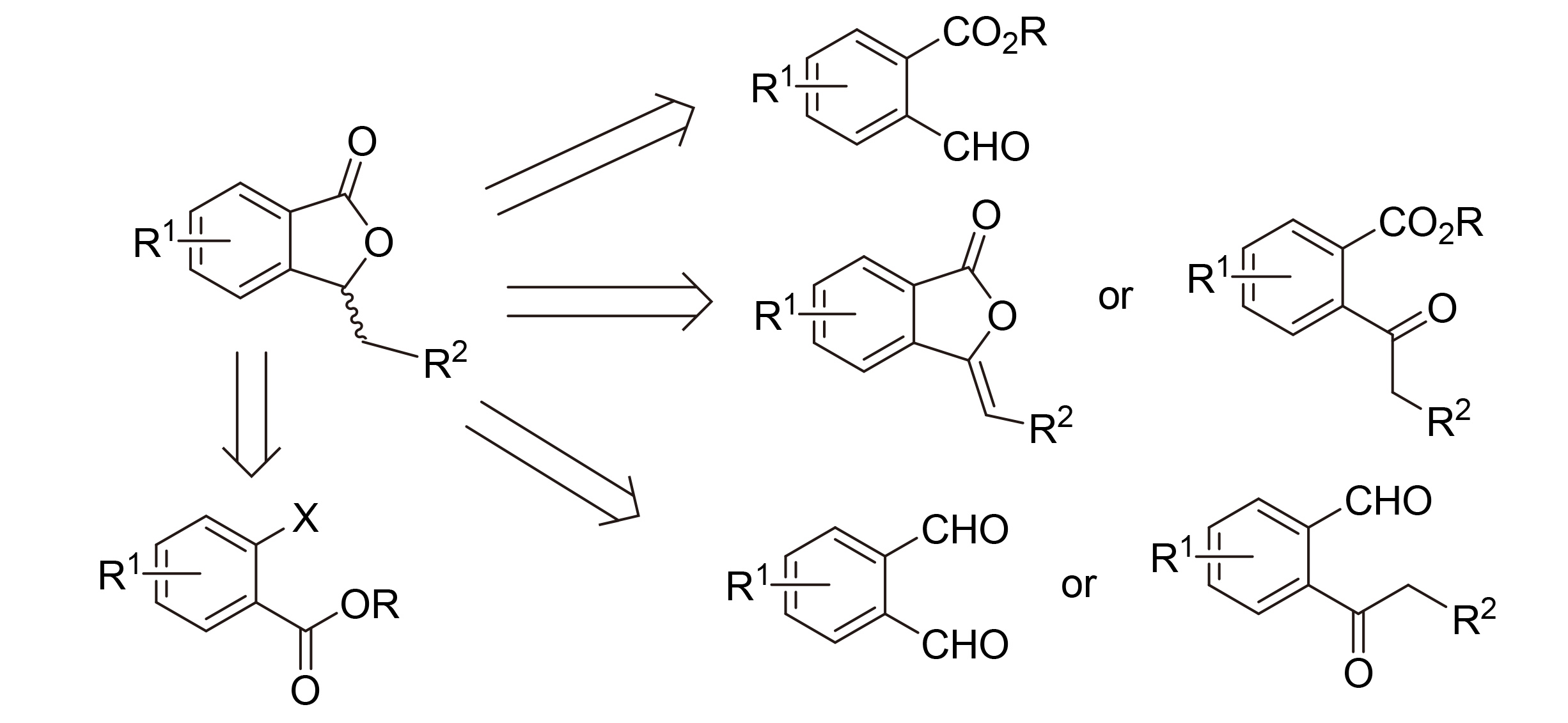
3-Substituted phthalides are widely distributed in plants and fungi. They are active ingredients in traditional Chinese herbal medicines, and have attracted much attention in modern medicinal chemistry. The synthetic methods of 3-substituted phthalides are reviewed, especially those in enantioselective manners. The main approach involves: (a) construction of lactones from C—C bond formation reactions, e.g. an aldol/lactonization cascade reaction of 2-acylbenzoates and alikes, (b) construction of lactones via C—O bond formation reactions, e.g. reductive lactonization of 2-acylbenzoates or reduction of 3-alkenyl phthalides, intramolecular oxidation/lactonization, or intramolecular redox/lactonization. These methods are of great significance for the high stereoselective synthesis of phthalides and drug research.
Key words: phthalide; isobenzofuran-1(3H)-one; synthesis; enantioselectivity; natural product
Quancheng Li , Lan Jiang , Rui Bai , Yongkang Han , Zhengning Li . Progress in the Synthesis of 3-Substituted Phthaides[J]. Chinese Journal of Organic Chemistry, 2021 , 41(9) : 3390 -3399 . DOI: 10.6023/cjoc202104063
| [1] | Atanasov, A. G.; Zotchev, S. B.; Dirsch, V. M.; Supuran, C. T. Nat. Rev. Drug Discovery. 2021, 20, 20. |
| [2] | Cragg, G. M.; Newman, D. J.; Snader, K. M. J. Nat. Prod. 1997, 60, 52. |
| [3] | Hon, P. M.; Lee, C. M.; Choang, T. F.; Chui, K. Y.; Wong, H. N. C. Phytochemistry 1990, 29, 1189. |
| [4] | Lin, G.; Chan, S. K.; Chung, H. S.; Li, S. L. Stud. Nat. Prod. Chem. 2005, 32, 611. |
| [5] | Zou, J.; Chen, G. D.; Zhao, H.; Huang, Y.; Luo, X.; Xu, W.; He, R. R.; Hu, D.; Yao, X. S.; Gao, H. Org. Lett. 2018, 20, 884. |
| [6] | Beck, J. J.; Chou, S.-C. J. Nat. Prod. 2007, 70, 891. |
| [7] | Brady, S. F.; Wagenaar, M. M.; Singh, M. P.; Janso, J. E.; Clardy, J. Org. Lett. 2000, 2, 4043. |
| [8] | Wang, X.-L.; Wang, Z.-Y.; Yin, J. Zhang, Y.-H. Prog. Pharm. 2016, 40, 89. (in Chinese). |
| [8] | ( 王晓丽, 王兆亚, 尹健, 张奕华, 药学进展, 2016, 40, 89.) |
| [9] | Anderson, W. K.; Boehm, T. L.; Makara, G. M.; Swann, R. T. J. Med. Chem. 1996, 39, 46. |
| [10] | Floryk, D.; Huberman, E. Cancer Lett. 2006, 231, 20. |
| [11] | Chapuis, A. G.; Paolo Rizzardi, G.; D'Agostino, C.; Attinger, A.; Knabenhans, C.; Fleury, S.; Acha-Orbea, H.; Pantaleo, G. Nat. Med. 2000, 6, 762. |
| [12] | Ban, H. S.; Lee, S.; Kim, Y. P.; Yamaki, K.; Shin, K. H.; Ohuchi, K. Biochem. Pharmacol. 2002, 64, 1345. |
| [13] | Batsuren, D.; Batirov, É. K.; Malikov, V. M.; Zemlyanskii, V. N.; Yagudaev, M. R. Chem. Nat. Compd. 1981, 17, 223. |
| [14] | Diao, X.; Deng, P.; Xie, C.; Li, X.; Zhong, D.; Zhang, Y.; Chen, X. Drug Metab. Dispos. 2013, 41, 430. |
| [15] | Wang, W.; Cha, X. X.; Reiner, J.; Gao, Y.; Qiao, H. L.; Shen, J. X.; Chang, J. B. Eur. J. Med. Chem. 2010, 45, 1941. |
| [16] | Kameda, K.; Namiki, M. Chem. Lett. 1974, 12, 1491. |
| [17] | Forgacs, P.; Provost, J.; Touche, A.; Jehanno, A. J. Nat. Prod. 1986, 49, 178. |
| [18] | Strobel, G.; Ford, E.; Worapong, J.; Harper, J. K.; Arif, A. M.; Grant, D. M.; Fung, P. C. W.; Chau, R. M. W. Phytochemistry 2002, 60, 179. |
| [19] | Arnone, A.; Assante, G.; Nasini, G.; Strada, S.; Vercesi, A. J. Nat. Prod. 2002, 65, 48. |
| [20] | Tada, M.; Chiba, K. Agric. Biol. Chem. 1984, 48, 1367. |
| [21] | Dai, Y.; Li, K.; She, J.; Zeng, Y.; Wang, H.; Liao, S.; Lin, X.; Yang, B.; Wang, J.; Tao, H.; Dai, H.; Zhou, X.; Liu, Y. Marine Drugs 2020, 18, 547. |
| [22] | Karmakar, R.; Pahari, P.; Mal, D. Chem. Rev. 2014, 114, 6213. |
| [23] | Li, L. Guangzhou Chem. Ind. 2017, 45, 26. (in Chinese). |
| [23] | ( 李磊, 广州化工, 2017, 45, 26.) |
| [24] | Jia, L.; Han, F. Beilstein J. Org. Chem. 2017, 13, 1425. |
| [25] | Limaye, R. A.; Kumbhar, V. B.; Natu, A. D.; Paradkar, M. V.; Honmore, V. S.; Chauhan, R. R.; Gample, S. P.; Sarkar, D. Bioorg. Med. Chem. Lett. 2013, 23, 711. |
| [26] | Maia, A. F. D.; Siqueira, R. P.; de Oliveira, F. M.; Ferreira, J. G.; da Silva, S. F.; Caiuby, C. A. D.; de Oliveira, L. L.; de Paula, S. O.; Souza, R. A. C.; Guilardi, S.; Bressan, G. C.; Teixeira, R. R. Bioorg. Med. Chem. Lett. 2016, 26, 2810. |
| [27] | Landge, S. M.; Berryman, M.; Toeroek, B. Tetrahedron Lett. 2008, 49, 4505. |
| [28] | Kore, R.; Srivastava, R. Catal. Commun. 2011, 12, 1420. |
| [29] | Palillero-Cisneros, A.; Bedolla-Medrano, M.; Ordonez, M. Tetrahedron 2018, 74, 4174. |
| [30] | Yuan, S.; Wang, S. X.; Chen, J. J.; Zhao, L. F.; Yu, B.; Liu, H. M. Chin. J. Org. Chem. 2018, 38, 309. (in Chinese). |
| [30] | ( 袁硕, 王四喜, 陈锦杰, 赵龙飞, 余斌, 刘宏民, 有机化学, 2018, 38, 309.) |
| [31] | Zhang, H. Y.; Zhang, S. L.; Liu, L.; Luo, G. S.; Duan, W. H.; Wang, W. J. Org. Chem. 2010, 75, 368. |
| [32] | Lin, H.; Sun, X. W. Tetrahedron Lett. 2008, 49, 5343. |
| [33] | Lin, H.; Han, K. S.; Sun, X. W.; Lin, G. Q. Chin. J. Org. Chem. 2008, 28, 1479. (in Chinese). |
| [33] | ( 林华, 韩京成, 孙兴文, 林国强, 有机化学, 2008, 28, 1479.) |
| [34] | Tang, H.; Zhang, X.; Song, A.; Zhang, Z. Mod. Res. Catal. 2012, 1, 11. |
| [35] | Guo, T.; Wang, H. J.; Cao, C. C.; Chen, K. H.; Liu, Y.; Zhang, P. K.; Zhao, Y. H.; Ma, Y. C. Eur. J. Org. Chem. 2020, 12, 3613. |
| [36] | Perillo, M.; Di Mola, A.; Filosa, R.; Palombi, L.; Massa, A. RSC Adv. 2014, 4, 4239. |
| [37] | Ray, S. K.; Sadhu, M. M.; Biswas, R. G.; Unhale, R. A.; Singh, V. K. Org. Lett. 2019, 21, 417. |
| [38] | Mirabdolbaghi, R.; Dudding, T. Tetrahedron 2013, 69, 3287. |
| [39] | Kumbhar, S. V.; Chen, C. RSC Adv. 2018, 8, 41355. |
| [40] | Xing, C. H.; Liao, Y. X.; He, P.; Hu, Q. S. Chem. Commun. 2010, 46, 3010. |
| [41] | Huang, H. Y.; Wang, Y. B.; Zong, H.; Song, L. Appl. Organomet. Chem. 2019, 33, 12. |
| [42] | Carlos, A. M. M.; Stieler, R.; Ludtke, D. S. Org. Biomol. Chem. 2019, 17, 283. |
| [43] | Zhang, Z. B.; Lu, Y. Q.; Duan, X. F. Synth.-Stuttgart 2011, 3435. |
| [44] | Rayabarapu, D. K.; Chang, H. T.; Cheng, C. H. Chem.-Eur. J. 2004, 10, 2991. |
| [45] | Chang, H. T.; Jeganmohan, M.; Cheng, C. H. Chem.-Eur. J. 2007, 13, 4356. |
| [46] | Mahendar, L.; Satyanarayana, G. J. Org. Chem. 2016, 81, 7685. |
| [47] | Fan, J.; Wang, P. M.; Wang, J. N.; Zhao, X.; Liu, Z. W.; Wei, J. F.; Shi, X. Y. Sci. China Chem. 2018, 61, 153. |
| [48] | Kattela, S.; de Lucca, E. C.; Correia, C. R. D. Chem.-Eur. J. 2018, 24, 17691. |
| [49] | Nguyen, T. V. Q.; Rodriguez-Santamaria, J. A.; Yoo, W. J.; Kobayashi, S. Green Chem. 2017, 19, 2501. |
| [50] | Yamamoto, Y.; Ishii, J. I.; Nishiyama, H.; Itoh, K. J. Am. Chem. Soc. 2005, 127, 9625. |
| [51] | Tanaka, K.; Nishida, G.; Wada, A.; Noguchi, K. Angew. Chem., Int. Ed. 2004, 43, 6510. |
| [52] | Tanaka, K.; Osaka, T.; Noguchi, K.; Hirano, M. Org. Lett. 2007, 9, 1307. |
| [53] | Kitamura, M.; Ohkuma, T.; Inoue, S.; Sayo, N.; Kumobayashi, H.; Akutagawa, S.; Ohta, T.; Takaya, H.; Noyori, R. J. Am. Chem. Soc. 1988, 110, 629. |
| [54] | Everaere, K.; Scheffler, J. L.; Mortreux, A.; Carpentier, J. F. Tetrahedron Lett. 2001, 42, 1899. |
| [55] | Zhang, B.; Xu, M. H.; Lin, G. Q. Org. Lett. 2009, 11, 4712. |
| [56] | Lu, B.; Zhao, M.; Ding, G.; Xie, X.; Jiang, L.; Ratovelomanana- Vidal, V.; Zhang, Z. ChemCatChem 2017, 9, 3989. |
| [57] | Xie, J. H.; Liu, X. Y.; Xie, J. B.; Wang, L. X.; Zhou, Q. L. Angew. Chem., Int. Ed. 2011, 50, 7329. |
| [58] | Ge, Y.; Han, Z. B.; Wang, Z.; Feng, C. G.; Zhao, Q.; Lin, G. Q.; Ding, K. L. Angew. Chem., Int. Ed. 2018, 57, 13140. |
| [59] | Kawaguchi, S.; Nakamura, K.; Yamaguchi, K.; Sato, Y.; Gonda, Y.; Nishioka, M.; Sonoda, M.; Nomoto, A.; Ogawa, A. Eur. J. Org. Chem. 2017, 2017, 5343. |
| [60] | Kawaguchi, S.; Gonda, Y.; Masuno, H.; Vu, H. T.; Yamaguchi, K.; Shinohara, H.; Sonoda, M.; Ogawa, A. Tetrahedron Lett. 2014, 55, 6779. |
| [61] | Matsuda, T.; Suzuki, K.; Abe, S.; Kirikae, H.; Okada, N. Tetrahedron 2015, 71, 9264. |
| [62] | Youn, S. W.; Song, H. S.; Park, J. H. Org. Biomol. Chem. 2014, 12, 2388. |
| [63] | Youn, S. W.; Song, H. S.; Park, J. H. Org. Lett. 2014, 16, 1028. |
| [64] | Hayat, S.; Rahman, A.; Choudhary, M. I.; Khan, K. M.; Bayer, E. Tetrahedron Lett. 2001, 42, 1647. |
| [65] | Gerbino, D. C.; Augner, D.; Slavov, N.; Schmalz, H. G. Org. Lett. 2012, 14, 2338. |
| [66] | Yang, J.; Yoshikai, N. J. Am. Chem. Soc. 2014, 136, 16748. |
| [67] | Phan, D. H. T.; Kim, B.; Dong, V. M. J. Am. Chem. Soc. 2009, 131, 15608. |
| [68] | Shirai, T.; Iwasaki, T.; Kanemoto, K.; Yamamoto, Y. Chem.-Asian J. 2020, 15, 1858. |
| [69] | Cabrera, J. M.; Tauber, J.; Krische, M. J. Angew. Chem., Int. Ed. 2018, 57, 1390. |
| [70] | Trost, B. M.; Rivers, G. T.; Gold, J. M. J. Org. Chem. 1980, 45, 1835. |
| [71] | Patterson, J. W. Tetrahedron 1993, 49, 4789. |
| [72] | Birch, A. J.; Wright, J. J. Aust. J. Chem. 1969, 22, 2635. |
| [73] | Mal, D.; Pahari, P.; De, S. R. Tetrahedron 2007, 63, 11781. |
| [74] | Kuethe, J. T.; Maloney, K. M. Tetrahedron 2013, 69, 5248. |
| [75] | Soriano, M. D. P. C.; Shankaraiah, N.; Santosa, L. S. Tetrahedron Lett. 2010, 51, 1770. |
/
| 〈 |
|
〉 |