

Chinese Journal of Organic Chemistry >
Recent Progress in the Construction of S—S, P—S and C—S Bonds Involving O2-Initiated Sulfur-Centered Radicals
Received date: 2021-05-10
Revised date: 2021-06-08
Online published: 2021-07-06
Supported by
Science and Technology Planning Project of Guangdong Province(2021A0505030069); 100 Young Talents Program of Guangdong University of Technology(220413506)
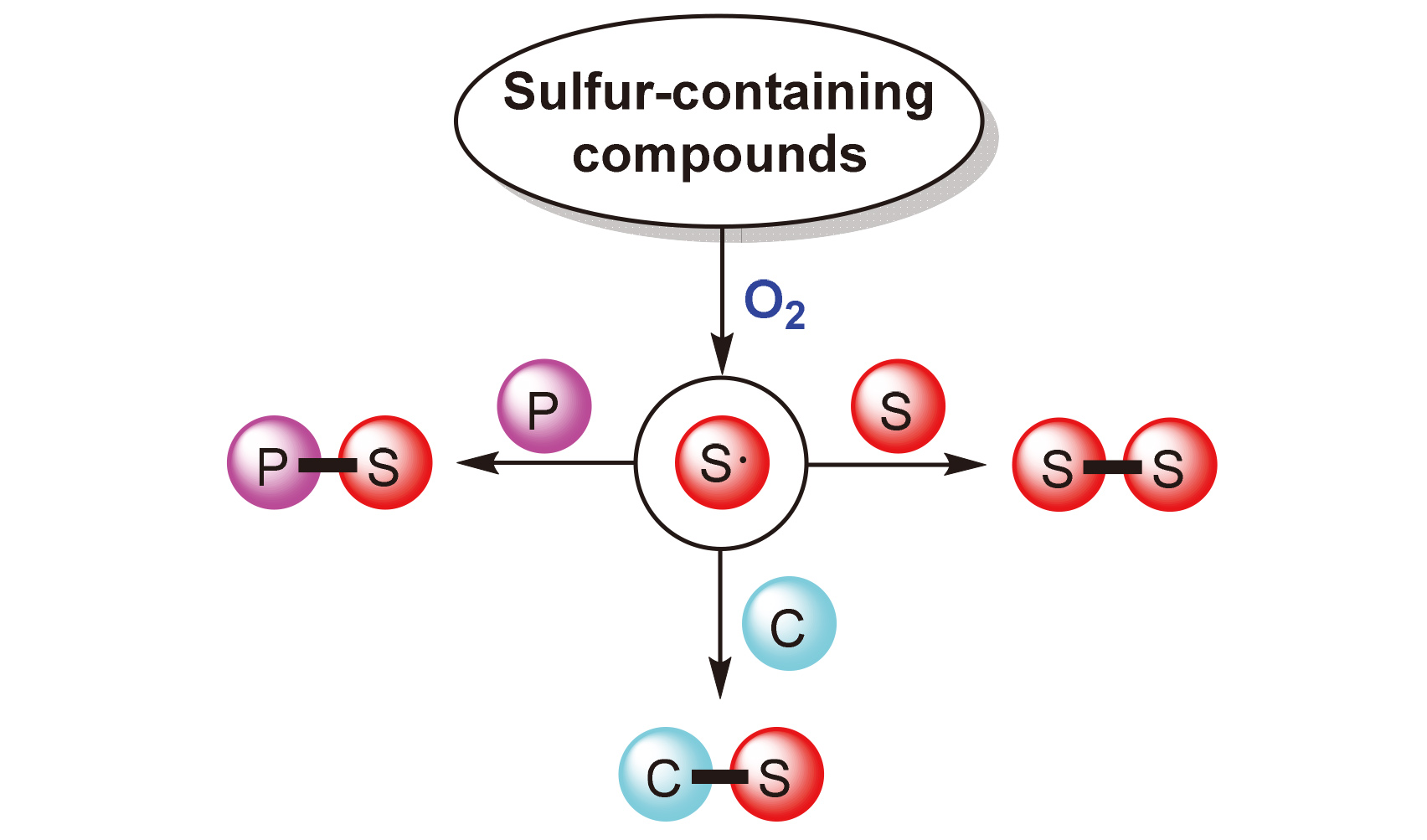
Sulfur-containing organic compounds have been widely used in organic synthesis, pharmaceuticals, pesticides and functional materials. Thus, the development of green, mild and highly efficient methodologies for the synthesis of organosulfur compounds is of great importance. Some sulfur-containing compounds can be directly initiated by O2 as the ideal green oxidant to generate sulfur-centered radicals, and the corresponding radical reactions provide a novel approach to the construction of S—S, P—S and C—S bonds. These reactions can be performed under mild conditions without the use of toxic reagents, transition metals and strong oxidants. In this review, the recent progress in the construction of S—S, P—S and C—S bonds involving O2-initiated sulfur-centered radicals is introduced on the basis of different reaction types.
Xi Zhao , Yingcong Ou , Yan Liu , Keiji Maruoka , Qian Chen . Recent Progress in the Construction of S—S, P—S and C—S Bonds Involving O2-Initiated Sulfur-Centered Radicals[J]. Chinese Journal of Organic Chemistry, 2021 , 41(9) : 3366 -3378 . DOI: 10.6023/cjoc202105015
| [1] | (a) Liu, H.; Jiang, X. Chem.-Asian J. 2013, 8, 2546. |
| [1] | (b) Lee, C.-F.; Liu, Y.-C.; Badsara, S. S. Chem.-Asian J. 2014, 9, 706. |
| [1] | (c) Chauhan, P.; Mahajan, S.; Enders, D. Chem. Rev. 2014, 114, 8807. |
| [1] | (d) Zhang, L.; Niu, C.; Yang, X.; Qin, H.; Yang, J.; Wen, J.; Wang, H. Chin. J. Org. Chem. 2020, 40, 1117. (in Chinese). |
| [1] | ( 张龙菲, 牛聪, 杨晓婷, 秦宏云, 杨建静, 文江伟, 王桦, 有机化学, 2020, 40, 1117.) |
| [1] | (e) Wang, B.; Zhou, Y.; Luo, S.; Luo, X.; Chen, W.; Yang, S.; Wang, Z. Chin. J. Org. Chem. 2021, 41, 171. (in Chinese). |
| [1] | ( 王柏文, 周永军, 罗时荷, 罗晓燕, 陈伟清, 杨诗敏, 汪朝阳, 有机化学, 2021, 41, 171.) |
| [2] | (a) Lin, Y.-M.; Lu, G.-P.; Wang, R.-K.; Yi, W.-B. Org. Lett. 2017, 19, 1100. |
| [2] | (b) Qiu, G; Lai, L.; Cheng, J.; Wu, J. Chem. Commun. 2018, 54, 10405. |
| [2] | (c) Hu, J.; Huang, Y.; Xu, X.; Qing, F. Chin. J. Org. Chem. 2019, 39, 177. (in Chinese). |
| [2] | ( 胡娟娟, 黄焰根, 徐修华, 卿凤翎, 有机化学, 2019, 39, 177.) |
| [2] | (d) Wang, J.-Y.; Ma, L.; Li, Y.; Wang, X.-S. Chin. J. Org. Chem. 2019, 39, 232. (in Chinese). |
| [2] | ( 王建勇, 马岚, 李彦, 王细胜, 有机化学, 2019, 39, 232.) |
| [2] | (e) Guo, W.; Tao, K.; Tan, W.; Zhao, M.; Zheng, L.; Fan, X. Org. Chem. Front. 2019, 6, 2048. |
| [2] | (f) Chen, Z.; Zhang, H.; Zhou, S.; Cui, X. Chin. J. Org. Chem. 2020, 40, 3866. (in Chinese). |
| [2] | ( 陈志超, 张红, 周树锋, 崔秀灵, 有机化学, 2020, 40, 3866.) |
| [2] | (g) Ghosh, A. K.; Mondal, S.; Hajra, A. Org. Lett. 2020, 22, 2771. |
| [2] | (h) Pramanik, M.; Choudhuri, K.; Mal, P. Org. Biomol. Chem. 2020, 18, 8771. |
| [2] | (i) Choudhuri, T.; Pramanik, M.; Mal, P. J. Org. Chem. 2020, 85, 11997. |
| [3] | (a) De?ne?s, F.; Schiesser, C. H.; Renaud, P. Chem. Soc. Rev. 2013, 42, 7900. |
| [3] | (b) De?ne?s, F.; Pichowicz, M.; Povie, G.; Renaud, P. Chem. Rev. 2014, 114, 2587. |
| [3] | (c) Pan, X.-Q.; Zou, J.-P.; Yi, W.-B.; Zhang, W. Tetrahedron 2015, 71, 7481. |
| [3] | (d) Yang, W.; Zhang, M.; Chen, W.; Yang, X.; Feng, J. Chin. J. Org. Chem. 2020, 40, 4060. (in Chinese). |
| [3] | ( 杨文超, 张明明, 陈旺, 杨小虎, 冯建国, 有机化学, 2020, 40, 4060.) |
| [4] | (a) Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. Chem. Soc. Rev. 2012, 41, 3381. |
| [4] | (b) Xu, J.; Song, Q. Chin. J. Org. Chem. 2016, 36, 1151. (in Chinese). |
| [4] | ( 许健, 宋秋玲, 有机化学, 2016, 36, 1151.) |
| [5] | (a) Chen, Q.; Huang, Y.; Wang, X.; Wu, J.; Yu, G. Org. Biomol. Chem. 2018, 16, 1713. |
| [5] | (b) Wen, C.; Wu, J.; Ou, Y.; Huang, Y.; Zhang, K.; Chen, Q. Tetrahedron Lett. 2018, 59, 3609. |
| [6] | Ruano, J. L. G.; Parra, A.; Alemán, J. Green Chem. 2008, 10, 706. |
| [7] | Liu, Y.; Wang, H.; Wang, C.; Wan, J.-P.; Wen, C. RSC Adv. 2013, 3, 21369. |
| [8] | Qiu, X.; Yang, X.; Zhang, Y.; Song, S.; Jiao, N. Org. Chem. Front. 2019, 6, 2220. |
| [9] | Song, S.; Zhang, Y.; Yeerlan, A.; Zhu, B.; Liu, J.; Jiao, N. Angew. Chem. Int. Ed. 2017, 56, 2487. |
| [10] | He, W.; Hou, X.; Li, X.; Song, L.; Yu, Q.; Wang, Z. Tetrahedron 2017, 73, 3133. |
| [11] | Wen, C.; Chen, Q.; Huang, Y.; Wang, X.; Yan, X.; Zeng, J.; Huo, Y.; Zhang, K. RSC Adv. 2017, 7, 45416. |
| [12] | Kharasch, M. S.; Nudenberg, W.; Mantell, G. J. J. Org. Chem. 1951, 16, 524. |
| [13] | Kamal, A.; Reddy, D. R.; Rajendar, J. Mol. Catal. A 2007, 272, 26. |
| [14] | Wang, H.; Lu, Q.; Qian, C.; Liu, C.; Liu, W.; Chen, K.; Lei, A. Angew. Chem. Int. Ed. 2016, 55, 1094. |
| [15] | Huo, C.; Wang, Y.; Yuan, Y.; Chen, F.; Tang, J. Chem. Commun. 2016, 52, 7233. |
| [16] | Wang, Y.; Jiang, W.; Huo, C. J. Org. Chem. 2017, 82, 10628. |
| [17] | Lu, Q.; Wang, H.; Peng, P.; Liu, C.; Huang, Z.; Luo, Y.; Lei, A. Org. Chem. Front. 2015, 2, 908. |
| [18] | Wang, H.; Wang, G.; Lu, Q.; Chiang, C.-W.; Peng, P.; Zhou, J.; Lei, A. Chem.-Eur. J. 2016, 22, 14489. |
| [19] | Lin, Y.-M.; Lu, G.-P.; Wang, R.-K.; Yi, W.-B. Org. Lett. 2016, 18, 6424. |
| [20] | Yu, G.; Ou, Y.; Chen, D.; Huang, Y.; Yan, Y.; Chen, Q. Synlett 2020, 31, 83. |
| [21] | Liu, Q.; Wang, L.; Yue, H.; Li, J.-S.; Luo, Z.; Wei, W. Green Chem. 2019, 21, 1609. |
| [22] | Chun, S.; Chung, J.; Park, J. E.; Chung, Y. K. ChemCatChem 2016, 8, 2476. |
| [23] | Choudhuri, K.; Mandal, A.; Mal, P. Chem. Commun. 2018, 54, 3759. |
| [24] | Liu, K.; Jia, F.; Xi, H.; Li, Y.; Zheng, X.; Guo, Q.; Shen, B.; Li, Z. Org. Lett. 2013, 15, 2026. |
| [25] | Sahoo, H.; Singh, S.; Baidya, M. Org. Lett. 2018, 20, 3678. |
| [26] | Lu, Q.; Zhang, J.; Wei, F.; Qi, Y.; Wang, H.; Liu, Z.; Lei, A. Angew. Chem. Int. Ed. 2013, 52, 7156. |
| [27] | Lu, Q.; Zhang, J.; Zhao, G.; Qi, Y.; Wang, H.; Lei, A. J. Am. Chem. Soc. 2013, 135, 11481. |
| [28] | Lu, Q.; Chen, J.; Liu, C.; Huang, Z.; Peng, P.; Wang, H.; Lei, A. RSC Adv. 2015, 5, 24494. |
| [29] | Shen, T.; Yuan, Y.; Song, S.; Jiao, N. Chem. Commun. 2014, 50, 4115. |
| [30] | Wei, W.; Liu, X.; Yang, D.; Dong, R.; Cui, Y.; Yuan, F.; Wang, H. Tetrahedron Lett. 2015, 56, 1808. |
| [31] | Liu, C.; Ding, L.; Guo, G.; Liu, W. Eur. J. Org. Chem. 2016, 2016, 910. |
| [32] | Liang, X.; Xiong, M.; Zhu, H.; Shen, K.; Pan, Y. J. Org. Chem. 2019, 84, 11210. |
| [33] | Liu, K.; Li, D.-P.; Zhou, S.-F.; Pan, X.-Q.; Shoberu, A.; Zou, J.-P. Tetrahedron 2015, 71, 4031. |
| [34] | Liao, Y.; Jiang, P.; Chen, S.; Xiao, F.; Deng, G.-J. RSC Adv. 2013, 3, 18605. |
| [35] | (a) Liu, X.; Cui, H.; Yang, D.; Dai, S.; Zhang, T.; Sun, J.; Wei, W.; Wang, H. RSC Adv. 2016, 6, 51830. |
| [35] | (b) Sun, P.; Yang, D.; Wei, W.; Jiang, L.; Wang, Y.; Dai, T.; Wang, H. Org. Chem. Front. 2017, 4, 1367. |
| [36] | Chen, Q.; Wang, X.; Wen, C.; Huang, Y.; Yan, X.; Zeng, J. RSC Adv. 2017, 7, 39758. |
| [37] | Jiang, Y.; Zou, J.-X.; Huang, L.-T.; Peng, X.; Deng, J.-D.; Zhu, L.-Q.; Yang, Y.-H.; Feng, Y.-Y.; Zhang, X.-Y.; Wang, Z. Org. Biomol. Chem. 2018, 16, 1641. |
| [38] | Chen, Q.; Huang, Y.; Wang, X.; Wen, C.; Yan, X.; Zeng, J. Tetrahedron Lett. 2017, 58, 3928. |
| [39] | Jiang, Y.; Deng, J.-d.; Wang, H.-h.; Zou, J.-x.; Wang, Y.-q.; Chen, J.-h.; Zhu, L.-q.; Zhang, H.-h.; Peng, X.; Wang, Z. Chem. Commun. 2018, 54, 802. |
| [40] | Zou, J.-X.; Wang, Y.-Q.; Huang, L.-T.; Jiang, Y.; Chen, J.-H.; Zhu, L.-Q.; Yang, Y.-H.; Feng, Y.-Y.; Peng, X.; Wang, Z. Org. Chem. Front. 2018, 5, 2317. |
| [41] | Chen, Q.; Yu, G.; Wang, X.; Huang, Y.; Yan, Y.; Huo, Y. Org. Biomol. Chem. 2018, 16, 4086. |
| [42] | Huang, L.-S.; Han, D.-Y.; Xu, D.-Z. Adv. Synth. Catal. 2019, 361, 4016. |
| [43] | Chen, Q.; Yu, G.; Wang, X.; Ou, Y.; Huo, Y. Green Chem. 2019, 21, 798. |
/
| 〈 |
|
〉 |