

Chinese Journal of Organic Chemistry >
N-Heterocyclic Carbene-Catalyzed [4+2] Annulation of Acetates and β-Silyl Enones: Highly Enantioselective Synthesis of β-Silyl δ-Lactones
Received date: 2021-05-01
Revised date: 2021-07-05
Online published: 2021-07-26
Supported by
National Natural Science Foundation of China(21602105)
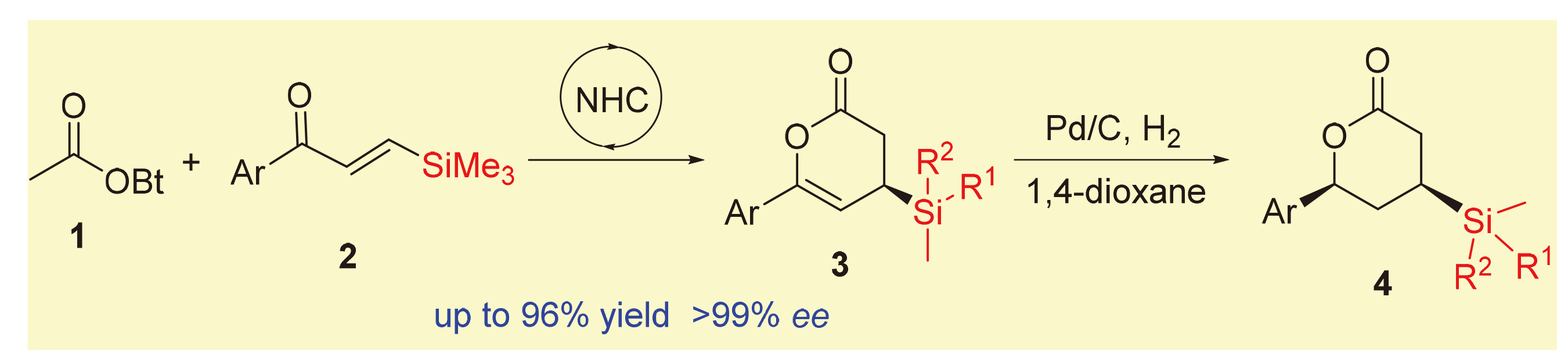
Asymmetric synthesis of chiral organosilanes with a potential application was realized by N-heterocyclic carbene- catalyzed [4+2] annulation of acetates and β-silyl enones. This strategy exhibits easy to obtain raw materials, mild reaction conditions, good substrate tolerance, simple operation and so on. Notably, excellent yield and enantioselectivity were also observed with expanding the reaction scale by 10 times. The target product showed excellent results in hydrogenation reduction. Moreover, the hypolipidemic drug ezetimibe was obtained through the synthetic transformation of the resulting products.
Yuxia Zhang , Jingcheng Guo , Jie Huang , Zhenqian Fu . N-Heterocyclic Carbene-Catalyzed [4+2] Annulation of Acetates and β-Silyl Enones: Highly Enantioselective Synthesis of β-Silyl δ-Lactones[J]. Chinese Journal of Organic Chemistry, 2021 , 41(11) : 4467 -4475 . DOI: 10.6023/cjoc202105002
| [1] | (a) Chan, T. H.; Wang, D. Chem. Rev. 1992, 92, 995. |
| [1] | (b) Masse, C. E.; Panek, J. S. Chem. Rev. 1995, 95, 1293. |
| [1] | (c) Fleming, I.; Barbero, A.; Walter, D. Chem. Rev. 1997, 97, 2063. |
| [1] | (d) Denmark, S. E.; Regens, C. S. Acc. Chem. Res. 2008, 41, 1486. |
| [1] | (e) Nakao, Y.; Hiyama, T. Chem. Soc. Rev. 2011, 40, 4893. |
| [1] | (f) Zhang, H. J.; Priebbenow, D. L.; Bolm, C. Chem. Soc. Rev. 2013, 42, 8540. |
| [1] | (g) Denmark, S. E.; Ambrosi, A. Org. Process Res. Dev. 2015, 19, 982. |
| [1] | (h) Komiyama, T.; Minami, Y.; Hiyama, T. ACS Catal. 2017, 7, 631. |
| [1] | (i) Cui, Y. M.; Lin, Y.; Xu, L. W. Coord. Chem. Rev. 2017, 330, 37. |
| [2] | (a) Patai, S.; Rappoport, Z.; Ojima, I. The Chemistry of Organic Silicon Compounds, Chichester, Wiley, 1989. |
| [2] | (b) Brook, M. A. Silicon in Organic, Organometallic, and Polymer Chemistry, New York, Wiley, 1999. |
| [3] | Cash, G. G. Pestic. Sci. 1997, 49, 29. |
| [4] | (a) Mutahi, M. W.; Nittoli, T.; Guo, L.; Sieburth, S. M. J. Am. Chem. Soc. 2002, 124, 7363. |
| [4] | (b) Bains, W.; Tacke, R. Curr. Opin. Drug Discovery Dev. 2003, 6, 526. |
| [4] | (c) Pooni, P. K.; Showell, G. A. Mini. Rev. Med. Chem. 2006, 6, 1169. |
| [4] | (d) Gately, S.; West, R. Drug Dev. Res. 2007, 68, 156. |
| [4] | (e) Franz, A. K.; Wilson, S. O. J. Med. Chem. 2012, 56, 388. |
| [4] | (f) Min, G. K.; Hernndez, D.; Skrydstrup, T. Acc. Chem. Res. 2013, 46, 457. |
| [5] | (a) Jensen, J. F.; Svendsen, B. Y.; la Cour, T. V.; Pedersen, H. L.; Johannsen, M. J. Am. Chem. Soc. 2002, 124, 4558. |
| [5] | (b) Tang, X.; Xie, L.; Chen, Y.; Tian, P.; Lin, G. Chin. J. Org. Chem. 2016, 36, 2011. (in Chinese) |
| [5] | (唐小齐, 谢立波, 陈雅丽, 田平, 林国强, 有机化学, 2016, 36, 2011.) |
| [5] | (c) Cheng, B.; Lu, P.; Zhang, H.; Cheng, X.; Lu, Z. J. Am. Chem. Soc. 2017, 139, 9439. |
| [5] | (d) Guo, J.; Shen, X.; Lu, Z. Angew. Chem. Int. Ed. 2017, 56, 615. |
| [5] | (e) Dai, Z.; Yu, Z.; Bai, Y.; Li, J.; Peng, J. Chin. J. Org. Chem. 2020, 40, 1177. (in Chinese) |
| [5] | (代自男, 余泽浩, 白赢, 厉嘉云, 彭家建, 有机化学, 2020, 40, 1177.) |
| [5] | (f) Liu, Y.; Dong, X.; Zhang, X. Chin. J. Org. Chem. 2020, 40, 1096. (in Chinese) |
| [5] | (刘元华, 董秀琴, 张绪穆, 有机化学, 2020, 40, 1096.) |
| [6] | (a) Zhang, Y. Z.; Zhu, S. F.; Wang, L. X.; Zhou, Q. L. Angew. Chem. Int. Ed. 2008, 120, 8624. |
| [6] | (b) Chen, D.; Zhu, D. X.; Xu, M. H. J. Am. Chem. Soc. 2016, 138, 1498. |
| [6] | (c) Zhang, H.; Li, L.; Shen, F.; Cai, T.; Shen, R. Chin. J. Org. Chem. 2020, 40, 873. (in Chinese) |
| [6] | (张慧苗, 李灵芝, 沈方旗, 蔡涛, 沈润溥, 有机化学, 2020, 40, 873.) |
| [7] | (a) Shintani, R.; Moriya, K.; Hayashi, T. J. Am. Chem. Soc. 2011, 133, 16440. |
| [7] | (b) Shintani, R.; Otomo, H.; Ota, K.; Hayashi, T. J. Am. Chem. Soc. 2012, 134, 7305. |
| [7] | (c) Zhang, Q.; An, K.; Liu, L. C.; Zhang, Q.; Guo, H.; He, W. Angew. Chem. Int. Ed. 2016, 55, 6319. |
| [7] | (d) Su, B.; Harting, J. F. J. Am. Chem. Soc. 2017, 139, 12137. |
| [8] | (a) Shintani, R.; Okamoto, K.; Hayashi, T. Org. Lett. 2005, 7, 4757. |
| [8] | (b) Balskus, E. P.; Jacobsen, E. N. J. Am. Chem. Soc. 2006, 128, 6810. |
| [8] | (c) Kacprzynski, M. A.; Kazane, S. A.; May, T. L.; Hoveyda, A. H. Org. Lett. 2007, 9, 3187. |
| [8] | (d) Zhao, K.; Loh, T. P. Chem.-Eur. J. 2014, 20, 16764. |
| [9] | (a) Chang, K. J.; Rayabarpu, D. K.; Yang, F. Y.; Cheng, C. H. J. Am. Chem. Soc. 2005, 127, 126. |
| [9] | (b) Ohmura, T.; Tamlguchi, H.; Suginome, M. J. Am. Chem. Soc. 2006, 128, 13682. |
| [9] | (c) Lee, K. S.; Hoveyda, A. H. J. Am. Chem. Soc. 2010, 132, 2898. |
| [9] | (d) Gilles, P.; Py, S. Org. Lett. 2012, 14, 1042. |
| [9] | (e) Pace, V.; Rae, J. P.; Harb, H. Y.; Procter, D. J. Chem. Commun. 2013, 49, 5150. |
| [9] | (f) Kitanosono, T.; Zhu, L.; Liu, C.; Xu, P.; Kobayashi, S. J. Am. Chem. Soc. 2015, 137, 15422. |
| [10] | O'brien, J. M.; Hoveyda, A. H. J. Am. Chem. Soc. 2011, 133, 7712. |
| [11] | (a) Zhang, Y. X.; Huang, J.; Guo, Y. Y.; Li, L.; Fu, Z. Q.; Huang, W. Angew. Chem. Int. Ed. 2018, 57, 4594. |
| [11] | (b) Zhang, Y. X.; Huang, X.; Guo, J. C.; Wei, C. L.; Gong, M. H.; Fu, Z. Q. Org. Lett. 2020, 22, 9545. |
| [12] | (a) Florence, G. J.; Gardner, N. M.; Paterson, I. Nat. Prod. Rep. 2008, 25, 342. |
| [12] | (b) Mondon, M.; Gesson, J. P. Curr. Org. Synth. 2006, 3, 41. |
| [12] | (c) Chiu, P.; Leung, L. T.; Ko, C. B. B. Nat. Prod. Rep. 2010, 27, 1066. |
| [13] | Selected reviews on NHC catalysis: (a) Enders, D.;,. Enders, D.; Balensiefer, T. Acc. Chem. Res. 2004, 37, 534. |
| [13] | (b) Enders, D.; Niemeier, O.; Henseler, A. Chem. Rev. 2007, 107, 5606. |
| [13] | (c) Nair, V.; Menon, R. S.; Biju, A. T.; Sinu, C. R.; Paul, R. R.; Jose, A.; Sreekumar, V. Chem. Soc. Rev. 2011, 40, 5336. |
| [13] | (d) Izquierdo, J.; Hutson, G. E.; Cohen, D. T.; Scheidt, K. A. Angew. Chem. Int. Ed. 2012, 51, 11686. |
| [13] | (e) Douglas, J.; Churchill, G.; Smith, A. D. Synthesis 2012, 44, 2295. |
| [13] | (f) Ryan, S. J.; Candish, L.; Lupton, D. W. Chem. Soc. Rev. 2013, 42, 4906. |
| [13] | (g) De Sarkar, S.; Biswas, A.; Samanta, R. C.; Studer, A. Chem.- Eur. J. 2013, 19, 4664. |
| [13] | (h) FHvre, M.; Pinaud, J.; Gnanou, Y.; Vignolle, J.; Taton, D. Chem. Soc. Rev. 2013, 42, 2142. |
| [13] | (i) Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485. |
| [13] | (j) Flanigan, D. M.; Romanov-Michailidis, F.; White, N. A.; Rovis, T. Chem. Rev. 2015, 115, 9307. |
| [13] | (k) Menon, R. S.; Biju, A. T.; Nair, V. Chem. Soc. Rev. 2015, 44, 5040. |
| [13] | (l) Menon, R. S.; Biju, A. T.; Nair, V. Beilstein J. Org. Chem. 2016, 12, 444. |
| [13] | (m) Zhang, C.; Hooper, J. F.; Lupton, D. W. ACS Catal. 2017, 7, 2583. |
| [13] | (n) Chen, X. Y.; Gao, Z. H.; Ye, S. Acc. Chem. Res. 2020, 53, 690. |
| [13] | (o) Ohmiya, H. ACS Catal. 2020, 10, 6862. |
| [13] | (p) Chen, X.; Wang, H.; Jin, Z.; Chi, Y. R. Chin. J. Chem. 2020, 38, 1167. |
| [14] | Hua, Y. Y.; Bin, H. Y.; Wei, T.; Cheng, H. A.; Lin, Z. P.; Fu, X. F.; Li, Y. Q.; Xie, J. H.; Yan, P. C.; Zhou, Q. L. Org. Lett. 2020, 22, 818. |
| [15] | (a) Brink, B. D.; DeFrancisco, J. R.; Hillner, J A.; Linton, B. R. J. Org. Chem. 2011, 76, 5258. |
| [15] | (b) Pradhan, N.; Paul, S.; Deka, S. J.; Roy, A.; trivedi, V.; Manna, D. ChemSelect 2017, 2, 5511. |
/
| 〈 |
|
〉 |