

Chinese Journal of Organic Chemistry >
Benzylic Oxidation Catalyzed by Cobalt(II)-Terpyridine Coordination Polymers
Received date: 2021-04-14
Revised date: 2021-06-10
Online published: 2021-08-10
Supported by
Hubei Provincial Scientific and Technological Innovation Team Project(T2020023); Science and Technology Plan Project of Jingmen City(2020ZDYF002)
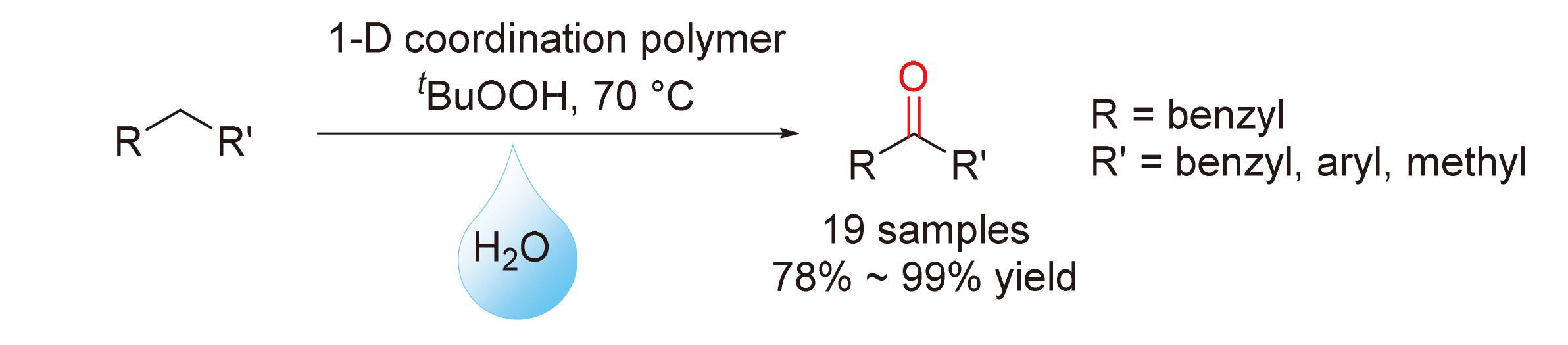
Direct benzylic C—H oxidation for the synthesis of aromatic ketones was developed. Employing cobalt(II)-terpyridine coordination polymers as catalyst, tert-butyl hydroperoxide (TBHP) as oxidant and Na2CO3 as activator, 19 aromatic ketones were prepared with good to excellent yields (78%~99%) in water. The reaction showed a broad range of substrates with good functional group tolerance and chemical selectivity. By control experiments, a trapping experiment using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) and detection of intermediates during reaction, a reasonable radical mechanism for this type of reaction was also demonstrated.
Jianqi Liu , Weiwei Fan , Hangxing Xiong , Jingyun Jiang , Hongju Zhan . Benzylic Oxidation Catalyzed by Cobalt(II)-Terpyridine Coordination Polymers[J]. Chinese Journal of Organic Chemistry, 2021 , 41(11) : 4409 -4414 . DOI: 10.6023/cjoc202104028
| [1] | Karimov, R. R.; Hartwig, J. F. Angew. Chem., Int. Ed. 2018, 57, 4234. |
| [2] | Wu, J.; Zhu, J.; Li, H.; Wu, C.; Shen, R.; Yu, L. Chin. J. Org. Chem. 2019, 39, 3328. (in Chinese) |
| [2] | (吴锦雯, 朱佳雯, 李慧, 吴春雷, 沈润溥, 余乐茂, 有机化学, 2019, 39, 3328.) |
| [3] | Sun, Q.; Sun, W. Chin. J. Org. Chem. 2020, 40, 3686. (in Chinese) |
| [3] | (孙强盛, 孙伟, 有机化学, 2020, 40, 3686.) |
| [4] | Ren, L.; Wang, L.; Lv, Y.; Li, G.; Gao, S. Org. Lett. 2015, 17, 2078. |
| [5] | Ren, L.; Gao, S. Chin. J. Org. Chem. 2017, 37, 1338. (in Chinese) |
| [5] | (任兰会, 高爽, 有机化学, 2017, 37, 1338.) |
| [6] | Pieber, B.; Kappe, C. O. Green Chem. 2013, 15, 320. |
| [7] | Bo, C.; Bu, Q.; Li, X.; Ma, G.; Wei, D.; Guo, C.; Dai, B.; Liu, N. J. Org. Chem. 2020, 85, 4324. |
| [8] | Peng, H.; Lin, A.; Zhang, Y.; Jiang, H.; Zhou, J.; Cheng, Y.; Zhu, C.; Hu, H. ACS Catal. 2012, 2, 163. |
| [9] | Li, H.; Li, Z.; Shi, Z. Tetrahedron 2009, 65, 1856. |
| [10] | Sterckx, H.; De Houwer, J.; Mensch, C.; Caretti, I.; Tehrani, K. A.; Herrebout, W. A.; Van Doorslaer, S.; Maes, B. U. W. Chem. Sci. 2016, 7, 346. |
| [11] | Hruszkewycz, D. P.; Miles, K. C.; Thiel, O. R.; Stahl, S. S. Chem. Sci. 2017, 8, 1282. |
| [12] | Miao, C.; Zhao, H.; Zhao, Q.; Xia, C.; Sun, W. Catal. Sci. Technol. 2016, 6, 1378. |
| [13] | Liu, P.; Liu, Y.; Wong, E. L.; Xiang, S.; Che, C. Chem. Sci. 2011, 2, 2187. |
| [14] | Shen, D.; Miao, C.; Wang, S.; Xia, C.; Sun, W. Org. Lett. 2014, 16, 1108. |
| [15] | Talsi, E. P.; Samsonenko, D. G.; Ottenbacher, R. V.; Bryliakov, K. P. ChemCatChem 2017, 9, 4580. |
| [16] | Urgoitia, G.; Sanmartin, R.; Herrero, M. T.; Domínguez, E. Chem. Commun. 2015, 51, 4799. |
| [17] | Yamazaki, S. Org. Lett. 1999, 1, 2129. |
| [18] | Muthupandi, P.; Sekar, G. Tetrahedron Lett. 2011, 52, 692. |
| [19] | Xia, J.; Cormier, K. W.; Chen, C. Chem. Sci. 2012, 3, 2240. |
| [20] | Jeong, Y.; Moon, Y.; Hong, S. Org. Lett. 2015, 17, 3252. |
| [21] | Li, P.; Wang, Y.; Wang, X.; Wang, Y.; Liu, Y.; Huang, K.; Hu, J.; Duan, L.; Hu, C.; Liu, J. J. Org. Chem. 2020, 85, 3101. |
| [22] | Yang, G.; Zhang, Q.; Miao, H.; Tong, X.; Xu, J. Org. Lett. 2005, 7, 263. |
| [23] | Alanthadka, A.; Devi, E. S.; Nagarajan, S.; Sridharan, V.; Suvitha, A.; Maheswari, C. U. Eur. J. Org. Chem. 2016, 2016, 4872. |
| [24] | Zhang, J.; Wang, Z.; Wang, Y.; Wan, C.; Zheng, X.; Wang, Z. Green Chem. 2009, 11, 1973. |
| [25] | Moriyama, K.; Takemura, M.; Togo, H. Org. Lett. 2012, 14, 2414. |
| [26] | Yin, L.; Wu, J.; Xiao, J.; Cao, S. Tetrahedron Lett. 2012, 53, 4418. |
| [27] | Sheldon, R. A. Chem. Soc. Rev. 2012, 41, 1437. |
| [28] | Waffel, D.; Alkan, B.; Fu, Q.; Chen, Y.; Schmidt, S.; Schulz, C.; Wiggers, H.; Muhler, M.; Peng, B. ChemPlusChem 2019, 84, 1155. |
| [29] | Zhang, G.; Zeng, H.; Li, S.; Johnson, J.; Mo, Z.; Neary, M. C.; Zheng, S. Dalton Trans. 2020, 49, 2610. |
| [30] | Zhang, G.; Li, S.; Wu, J.; Zeng, H.; Mo, Z.; Davis, K.; Zheng, S. Org. Chem. Front. 2019, 6, 3228. |
| [31] | Fan, W.; Li, L.; Zhang, G. J. Org. Chem. 2019, 84, 5987. |
| [32] | Zhang, G.; Wu, J.; Li, S.; Cass, S.; Zheng, S. Org. Lett. 2018, 20, 7893. |
| [33] | Wu, J.; Zeng, H.; Cheng, J.; Zheng, S.; Golen, J. A.; Manke, D. R.; Zhang, G. J. Org. Chem. 2018, 83, 9442. |
| [34] | Tan, J.; Zheng, T.; Yu, Y.; Xu, K. RSC Adv. 2017, 7, 15176. |
| [35] | Zhang, Z.; Li, J.; Yao, Y.; Sun, S. Cryst. Growth Des. 2015, 15, 5028. |
| [36] | Azarkamanzad, Z.; Farzaneh, F.; Maghami, M.; Simpson, J. New J. Chem. 2019, 43, 12020. |
| [37] | Han, X.; Zhou, Z.; Wan, C.; Xiao, Y.; Qin, Z. Synthesis 2013, 45, 615. |
| [38] | Zhang, J.; Biradar, A. V.; Pramanik, S.; Emge, T. J.; Asefa, T.; Li, J. Chem. Commun. 2012, 48, 6541. |
| [39] | Asgharpour, Z.; Farzaneh, F.; Abbasi, A. RSC Adv. 2016, 6, 95729. |
| [40] | Bryant, J. R.; Mayer, J. M. J. Am. Chem. Soc. 2003, 125, 10351. |
| [41] | Wang, T.; Jing, X.; Chen, C.; Yu, L. J. Org. Chem. 2017, 82, 9342. |
| [42] | Cheng, K.; Zhao, B.; Qi, C. RSC Adv. 2014, 4, 48698. |
| [43] | Li, L.; Liu, Z.; Tang, S.; Li, J.; Ren, X.; Yang, G.; Li, H.; Yuan, B. Catal. Commun. 2019, 127, 34. |
| [44] | Zhang, X.; Ji, X.; Su, R.; Weeks, B. L.; Zhang, Z.; Deng, S. ChemPlusChem 2013, 78, 703. |
/
| 〈 |
|
〉 |