

Chinese Journal of Organic Chemistry >
Synthesis, Antibacterial and Antifungal Evaluation of Rhodanine Derivatives Bearing Quinoxalinyl Imidazole Moiety as ALK5 Inhibitors
Received date: 2021-06-07
Revised date: 2021-07-12
Online published: 2021-08-10
Supported by
National Natural Science Foundation of China(8206023)
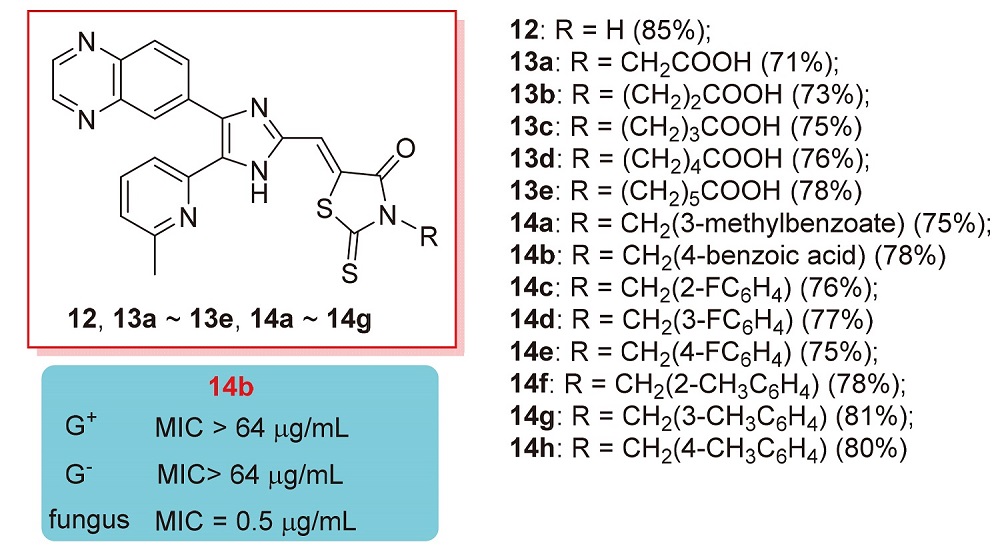
Transforming growth factor-β (TGF-β) is overexpressed in many diseases, and is an important target for treating tumors. Two series of 3-substituted-5-((5-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)-1H-imidazol-2-yl)methylene)-2-thioxo- thiazolidin-4-ones (12, 13a~13e, and 14a~14h) were synthesized and evaluated for their activin receptor-like kinase 5 (ALK5) inhibition activity. Among these compounds, (Z)-6-(5-((5-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)-1H-imidazol- 2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)hexanoic acid (13e) showed the highest activity (IC50=0.451 µmol•L–1) against ALK5 kinase, which had a good selectivity index of >22 against p38α MAP kinase, with 5.0-fold more selectivity than the clinical candidate of LY-2157299. These rhodanine compounds showed good antifungal activity and high selectivity against both Gram-positive and Gram-negative bacteria. These compounds showed similar or higher antifungal activity (MIC=0.5 or 1 µg/mL) to the positive control compound fluconazole (MIC=1 µg/mL).
Key words: rhodanine; TGF-β; quinoxalinyl imidazole; ALK5 inhibitor; antimicrobial; antifungal
Lizhuo Han , Limin Zhao , Huimin Wang , Tong Dou , Fangyan Guo , Junda Qi , Wenbo Xu , Lianxun Piao , Xuejun Jin , Fen'er Chen , Huri Piao , Changji Zheng , Chenghua Jin . Synthesis, Antibacterial and Antifungal Evaluation of Rhodanine Derivatives Bearing Quinoxalinyl Imidazole Moiety as ALK5 Inhibitors[J]. Chinese Journal of Organic Chemistry, 2021 , 41(11) : 4428 -4436 . DOI: 10.6023/cjoc202106015
| [1] | Jin, C. H.; Krishnaiah, M.; Sreenu, D.; Rao, K. S.; Subrahmanyam, V. B.; Park, C. Y.; Son, J. Y.; Sheen, Y. Y.; Kim, D. K. Bioorg. Med. Chem. 2011, 19, 2633. |
| [2] | Ma, J.; Mi, C.; Wang, K. S.; Lee, J. J.; Jin, X. J. Pharmacol. Sci. 2016, 130, 43. |
| [3] | Zhang, Z. H.; Mi, C.; Wang, K. S.; Wang, Z.; Li, M. Y.; Zuo, H. X.; Xu, G. H.; Li, X.; Piao, L. X.; Ma, J.; Jin, X. Phytother. Res. 2018, 32, 65. |
| [4] | Shang, Y.; Han, X.; Yao, Y. L.; Li, Y. M.; Zhang, J.; Shao, D. Y.; Hou, L. S.; Fan, Y.; Song, S. Z.; Lian, L. H.; Nan, J. X.; Wu, Y. L. Biomed. Pharmacother. 2018, 107, 374. |
| [5] | Lu, Y.; Xu, X.; Jiang, T.; Jin, L.; Zhao, X. D.; Cheng, J. H.; Jin, X. J.; Ma, J. Piao, H. N.; Piao, L. X. Int. Immunopharmacol. 2019, 67, 119. |
| [6] | Xing, Y.; Mi, C.; Wang, Z.; Zhang, Z. H.; Li, M.; Zuo, H. X.; Wang, J. Y.; Jin, X.; Ma, J. Pharmacol. Res. 2018, 135, 166. |
| [7] | Wang, Z.; Li, M. Y.; Zhang, Z. H.; Zuo, H. X.; Wang, J. Y.; Xing, Y.; Ri, M. H.; Jin, H. L.; Jin, C. H.; Xu, G. H.; Piao, L. X.; Jiang, C. G.; Ma, J.; Jin, X. Pharmacol. Res. 2020, 155, 104727. |
| [8] | Cao, Y. P.; Pan, M.; Song, Y. L.; Zhang, H. L.; Su, H. T.; Shan, B. C.; Piao, H. X. Eur. Rev. Med. Pharmacol. 2019, 23, 7863. |
| [9] | Chen, S.; Zhang, Q.; Xu, D.; Li, Y.; Fan, Y.; Li, W.; Yin, X.; Zhang, Y.; Liu, J.; Li, X.; Sun, L.; Jin, N. Anti-cancer Drug 2018, 29, 197. |
| [10] | Wu, Y. L.; Zhang, Y. J.; Yao, Y. L.; Li, Z. M.; Han, X.; Lian, L. H.; Zhao, Y. Q.; Nan, J. X. Toxicol. Lett. 2016, 258, 147. |
| [11] | Zhu, W. J.; Cui, B. W.; Wang, H. M.; Nan, J. X.; Piao, H. R.; Lian, L. H.; Jin, C. H. Eur. J. Med. Chem. 2019, 180, 15. |
| [12] | Guo, Z.; Song, X.; Zhao, L. M.; Piao, M. G.; Quan, J.; Piao, H. R.; Jin, C. H. Bioorg. Med. Chem. Lett. 2019, 29, 2070. |
| [13] | Li, Y. W.; Li, X. Y.; Li, S.; Zhao, L. M.; Ma, J.; Piao, H. R.; Jiang, Z.; Jin, C. H.; Jin, X. Bioorg. Med. Chem. Lett. 2020, 30, 126822. |
| [14] | Derynck, R.; Zhang, Y. E. Nature 2003, 425, 577. |
| [15] | Jin, C. H.; Krishanaiah, M.; Sreenu, D.; Subrahmanyam, V. B.; Park, H. J.; Park, S. J.; Sheen, Y. Y.; Kim, D. K. Bioorg. Med. Chem. 2014, 22, 2724. |
| [16] | Brandes, A. A.; Carpentier, A. F.; Kesari, S.; Sepulveda-Sanchez, J. M.; Wheeler, H. R.; Chinot, O.; Cher, L.; Steinbach, J. P.; Capper, D.; Specenier, P.; Rodon, J.; Cleverly, A.; Smith, C.; Gueorguieva, I.; Miles, C.; Guba, S. C.; Desaiah, D.; Lahn, M. M.; Wick, W. A. Neuro. Oncol. 2016, 18, 1146. |
| [17] | Battle, E.; Massague, J. Immunity 2019, 50, 924. |
| [18] | Jin, C. H.; Krishnaiah, M.; Sreenu, D.; Subrahmanyam, V. B.; Rao, K. S.; Lee, H. J.; Park, S. J.; Park, H. J.; Lee, K.; Sheen, Y. Y.; Kim, D. K. J. Med. Chem. 2014, 57, 4213. |
| [19] | Jin, C. H.; Sreenu, D.; Krishnaiah, M.; Subrahmanyam, V. B.; Rao, K. S.; Mohan, A. V. N.; Park, C. V.; Son, J. Y.; Son, D. H.; Park, H. J.; Sheen, Y. Y.; Kim, D. K. Eur. J. Med. Chem. 2011, 46, 3917. |
| [20] | Zhao, L. M.; Guo, Z.; Xue, Y. J.; Min, J. Z.; Zhu, W. J.; Li, X. Y.; Piao, H. R.; Jin, C. H. Molecules 2018, 23, 3369. |
| [21] | Patel, H. M.; Sing, B.; Bhardwaj, V.; Palkar, M.; Shaikh, M. S.; Rane, R.; Alwan, W. S.; Gadad, A. K.; Noolvi, M. N.; Karpoormath, R. Eur. J. Med. Chem. 2015, 93, 599. |
| [22] | Devi, P. B.; Samala, G.; Sridevi, J. P.; Saxena, S.; Alvala, M.; Salina, E. G.; Sriam, D.; Yogeeswari, P. Chem. Med. Chem. 2014, 9, 2538. |
| [23] | Ottanà, R.; Paoli, P.; Naß, A.; Lori, G.; Gardile, V.; Adornato, I.; Rotondo, A.; Graziano, A. C. E.; Wolber, G.; Maccari, R. Eur. J. Med. Chem. 2017, 127, 840. |
| [24] | Liang, Y.; Tang, M. L.; Huo, Z.; Zhang, C.; Sun, X. Molecules 2020, 25, 1138. |
| [25] | Chen, Z. H.; Zheng, C. J.; Sun, L. P.; Piao, H. R. Eur. J. Med. Chem. 2010, 45, 5739. |
| [26] | Khodair, A. I.; Awad, M. K.; Gesson, J. P.; Elshaier, Y. A. M. M. Carbohydr. Res. 2020, 487, 107894. |
| [27] | Russell, A. J.; Westwood, I. M.; Crawford, M. H. J.; Robinson, J.; Kawamura, A.; Redfield, C.; Laurieri, N.; Lowe, E. D.; Davies, S. G.; Sim, E. Bioorg. Med. Chem. 2009, 17, 905. |
| [28] | Gandini, A.; Bartolini, M.; Tedesco, D.; Martinez-Gonzalez, L.; Roca, C.; Campillo, N. E.; Zaldivar-Diez, J.; Perez, C.; Zuccheri, G.; Miti, A.; Feoli, A.; Castellano, S.; Petralla, S.; Petralla, S.; Monti, B.; Rossi, M.; Moda, F.; Legname, G.; Martinez, A.; Bolognesi, M. L. J. Med. Chem. 2018, 61, 7640. |
| [29] | Song, M. X.; Zheng, C. J.; Deng, X. Q.; Wang, Q.; Hou, S. P.; Liu, T. T.; Xing, X. L.; Piao, H. R. Eur. J. Med. Chem. 2012, 54, 403. |
| [30] | Guo, M.; Zheng, C. J.; Song, M. X.; Wu, Y.; Sun, L. P.; Li, Y. J.; Liu, Y.; Piao, H. R. Bioorg. Med. Chem. Lett. 2013, 23, 4358. |
| [31] | Song, M. X.; Zheng, C. J.; Deng, X. Q.; Sun, L. P.; Wu, Y.; Hong, L.; Li, Y. J.; Liu, Y.; Wei, Z. Y.; Jin, M. J.; Piao, H. R. Eur. J. Med. Chem. 2013, 60, 376. |
| [32] | Wei, Z. Y.; Liu, J. C.; Zhang, W.; Li, Y. R.; Li, C.; Zheng, C. J.; Piao, H. R. Med. Chem. 2016, 12, 751. |
| [33] | Liu, X. F.; Zheng, C. J.; Sun, L. P.; Liu, X. K.; Piao, H. R. Eur. J. Med. Chem. 2011, 46, 3469. |
| [34] | Hardej, D.; Ashby, Jr C. R.; Khadtare, N. S.; Kulkarni, S. S.; Singh, S.; Talele, T. T.; Eur. J. Med. Chem. 2010, 45, 5827. |
| [35] | Zhang, T. Y.; Li, C.; Tian, Y. S.; Li, J. J.; Sun, L. P.; Zheng, C. J.; Piao, H. R. Chin. Chem. Lett. 2017, 58, 1737. |
| [36] | Zuo, H. X.; Jin, Y.; Wang, Z.; Li, M. Y.; Zhang, Z. H.; Wang, J. Y.; Xing, Y.; Ri, M. H.; Jin, C. H.; Xu, G. H.; Piao, L. X.; Ma, J.; Jin, X. J. Ethnopharmacol. 2020, 257, 112835. |
| [37] | Xu, X.; Jin, L.; Jiang, T.; Lu, Y.; Aosai, F.; Piao, H. N.; Xu, G. H.; Jin, C. H.; Jin, X. J.; M. J.; Piao, L. X.; J. Ginseng Res. 2020, 44, 704. |
| [38] | Kim, D. K.; Jung, S. H.; Lee, H. S.; Dewang, P. M. Eur. J. Med. Chem. 2009, 44, 568. |
| [39] | Kim, D. K.; Jang, Y.; Lee, H. S.; Park, H. J.; Yoo, J. J. Med. Chem. 2007, 50, 3143. |
| [40] | Xu, G.; Zhang, Y.; Wang, H.; Guo, Z.; Wang, X.; Li, X.; Chang, S.; Sun, T.; Yu, Z.; Xu, T.; Zhao, L.; Wang, Y.; Yu, W. Eur. J. Med. Chem. 2020, 198, 112354. |
/
| 〈 |
|
〉 |