

Chinese Journal of Organic Chemistry >
Copper-Catalyzed Intramolecular Dearomative Arylation of Naphthylamines
Received date: 2021-05-29
Revised date: 2021-07-31
Online published: 2021-08-19
Supported by
National Natural Science Foundation of China(21702184); National Natural Science Foundation of China(22071217); National Natural Science Foundation of China(91956117)
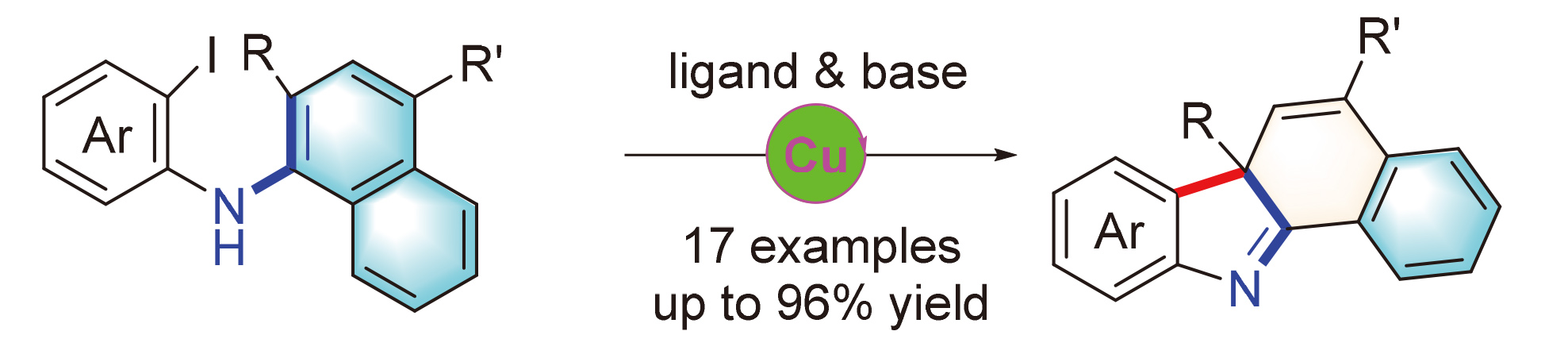
A CuI-catalyzed intramolecular dearomative arylation of naphthylamines is developed through a Ullmann-type cross-coupling reaction, which led to a range of fused 3H-indole derivatives in good to excellent yields.
Key words: Ullmann coupling; naphthylamine; arylation; dearomatization
Jun Lu , Renxiao Liang , Yixia Jia . Copper-Catalyzed Intramolecular Dearomative Arylation of Naphthylamines[J]. Chinese Journal of Organic Chemistry, 2021 , 41(10) : 4007 -4013 . DOI: 10.6023/cjoc202105050
| [1] | (a) Zheng, C.; You, S.-L. ACS Cent. Sci. 2021, 7, 432. |
| [1] | (b) Sharma, U. K.; Ranjan, P.; Van der Eycken, E. V.; You, S.-L. Chem. Soc. Rev. 2020, 49, 8721. |
| [1] | (c) Huang, G.; Yin, B. Adv. Synth. Catal. 2019, 361, 405. |
| [1] | (d) Wu, W.-T.; Zhang, L.; You, S.-L. Chem. Soc. Rev. 2016, 45, 1570. |
| [1] | (e) Zheng, C.; You, S.-L. Chem 2016, 1, 830. |
| [1] | (f) Zhuo, C.-X.; Zheng, C.; You, S.-L. Acc. Chem. Res. 2014, 47, 2558. |
| [1] | (g) Zhuo, C.-X.; Zhang, W.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 1266. |
| [1] | (h) Li, K.; Bai, L.; Luan, X. Chin. J. Org. Chem. 2019, 39, 2211. (in Chinese) |
| [1] | (李锟雨, 白璐, 栾新军, 2019, 39, 2211.) |
| [1] | (i) Yan, Q.; Fan, R.; Liu, B.; Su, S.; Wang, B.; Yao, T.; Tan, J. Chin. J. Org. Chem. 2021, 41, 455. (in Chinese) |
| [1] | (闫强, 范荣, 刘斌斌, 苏帅松, 王勃, 姚团利, 谭嘉靖, 有机化学, 2021, 41, 455.) |
| [2] | (a) Bai, Y.; Liu, A.; Wu, X.-X.; Chen, S.; Wang, J. J. Org. Chem. 2020, 85, 6687. |
| [2] | (b) Hu, J.; Pan, S.; Zhu, S.; Yu, P.; Xu, R; Zhong, G.; Zeng, X. J. Org. Chem. 2020, 85, 7896. |
| [2] | (c) Wu, X.-X.; Liu, A.; Mou, M.; Chen, H.; Chen, S. J. Org. Chem. 2018, 83, 14181. |
| [2] | (d) Fan, L.; Liu, J.; Bai, L.; Wang, Y.; Luan, X. Angew. Chem., Int. Ed. 2017, 56, 14257. |
| [2] | (e) Luo, L.; Zheng, H.; Liu, J.; Wang, H.; Wang, Y.; Luan, X. Org. Lett. 2016, 18, 2082. |
| [2] | (f) Nemoto, T., Nozaki, T.; Yoshida, M.; Hamada, Y. Adv. Synth. Catal. 2013, 355, 2693. |
| [2] | (g) Nemoto, T.; Zhao, Z. D.; Yokosaka, T.; Suzuki, Y.; Wu, R.; Hamada, Y. Angew. Chem., Int. Ed. 2013, 52, 2217. |
| [2] | (h) Yoshida, M.; Nemoto, T.; Zhao, Z.; Ishige, Y.; Hamada, Y. Tetrahedron: Asymmetry 2012, 23, 859. |
| [2] | (i) Rousseaux, S.; Fortanet, J. G.; Sanchez, M. A. D. A.; Buchwald, S. L. J. Am. Chem. Soc. 2011, 133, 9282. |
| [2] | (j) Wu, Q.-F.; Liu, W.-B.; Zhuo, C.-X.; Rong, Z.-Q.; Ye, K.-Y.; You, S.-L. Angew. Chem., Int. Ed. 2011, 50, 4455. |
| [2] | (k) Nemoto, T.; Ishige, Y.; Yoshida, M.; Kohno, Y.; Kanematsu, M.; Hamada, Y. Org. Lett. 2010, 12, 5020. |
| [3] | (a) Bedford, R. B.; Fey, N.; Haddow, M. F.; Sankey, R. F. Chem. Commun. 2011, 47, 3649. |
| [3] | (b) Bedford, R. B.; Butts, C. P.; Haddow, M. F.; Osborne, R.; Sankey, R. F. Chem. Commun. 2009, 4832. |
| [4] | (a) Fu, Z.; Zhu, J.; Guo, S.; Lin, A. Chem. Commun. 2021, 57, 1262. |
| [4] | (b) Yamaguchi, M.; Fujiwara, S.; Manabe, K. Org. Lett. 2019, 21, 6972. |
| [4] | (c) Ding, L.; Gao, R.-D.; You, S. L. Chem. Eur. J. 2019, 25, 4330. |
| [4] | (d) Fang, X.; Li, Q.; Shi, R.; Yao, H.; Lin, A. Org. Lett. 2018, 20, 6084. |
| [4] | (e) Trost, B. M.; Bai, W.-J.; Hohn, C.; Bai, Y.; Cregg, J. J. Am. Chem. Soc. 2018, 140, 6710. |
| [4] | (f) Gao, R.-D.; Ding, L.; Zheng, C.; Dai, L.-X.; You, S.-L. Org. Lett. 2018, 20, 748. |
| [4] | (g) Gao, S.; Wu, Z.; Fang, X.; Lin, A.; Yao, H. Org. Lett. 2016, 18, 3906. |
| [4] | (h) Gao, R.-D.; Xu, Q.-L.; Zhang, B.; Gu, Y.; Dai, L.-X.; You, S.-L. Chem. Eur. J. 2016, 22, 11601. |
| [4] | (i) Zhang, H.; Hu, R.-B.; Liu, N.; Li, S.-X.; Yang, S.-D. Org. Lett. 2016, 18, 28. |
| [4] | (j) Zhuo, C.-X.; Cheng, Q.; Liu, W.-B.; Zhao, Q.; You, S.-L. Angew. Chem., Int. Ed. 2015, 54, 8475. |
| [4] | (k) Gao, R.-D.; Liu, C.; Dai, L.-X.; Zhang, W.; You, S.-L. Org. Lett. 2014, 16, 3919. |
| [4] | (l) Yang, Z.-P.; Zhuo, C.-X.; You, S.-L. Adv. Synth. Catal. 2014, 356, 1731. |
| [4] | (m) Zhang, X.; Han, L.; You, S.-L. Chem. Sci. 2014, 5, 1059. |
| [4] | (n) Zhang, X.; Liu, W.-B.; Wu, Q.-F.; You, S.-L. Org. Lett. 2013, 15, 3746. |
| [4] | (o) Montgomery, T. D.; Zhu, Y.; Kagawa, N.; Rawal, V. H. Org. Lett. 2013, 15, 1140. |
| [4] | (p) Wu, K.-J.; Dai, L.-X.; You, S.-L. Org. Lett. 2012, 14, 3772. |
| [4] | (q) Wu, Q.-F.; Zheng, C.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 1680. |
| [4] | (r) Wu, K.-J.; Dai, L.-X.; You, S.-L. Org. Lett. 2012, 14, 3772. |
| [4] | (s) Trost, B. M.; Quancard, J. J. Am. Chem. Soc. 2006, 128, 6314. |
| [4] | (t) Kimura, M.; Futamata, M.; Mukai, R.; Tamaru, Y. J. Am. Chem. Soc. 2005, 127, 4592. |
| [5] | (a) Liang, R.-X.; Song, L.-J.; Lu, J.-B.; Xu, W.-Y.; Ding, C.; Jia, Y.-X. Angew. Chem., Int. Ed. 2021, 60, 7412. |
| [5] | (b) Zhou, B.; Wang, H.; Cao, Z.-Y.; Zhu, J.-W.; Liang, R.-X.; Jia, Y.-X. Nat. Commun. 2020, 11, 4380. |
| [5] | (c) Liang, R.-X.; Wang, K.; Wu, Q.; Sheng, W.-J.; Jia, Y.-X. Organometallics 2019, 38, 3927. |
| [5] | (d) Weng, J.-Q.; Xing, L.-L.; Hou, W.-R.; Liang, R.-X.; Jia, Y.-X. Org. Chem. Front. 2019, 6, 1577. |
| [5] | (e) Marchese, A. D.; Lind, F.; MahonÁ, E.; Yoon, H.; Lautens, M. Angew. Chem., Int. Ed. 2019, 58, 5095. |
| [5] | (f) Liang, R.-X.; Wang, K.; Song, L.-J.; Sheng, W.-J.; Jia, Y.-X. RSC Adv. 2019, 9, 13959. |
| [5] | (g) Shen, C.; Zeidan, N.; Wu, Q.; Breuers, C. B. J.; Liu, R.-R.; Jia, Y.-X.; Lautens, M. Chem. Sci. 2019, 10, 3118. |
| [5] | (h) Li, X.; Zhou, B.; Yang, R.-Z.; Yang, F.-M.; Liang, R.-X.; Liu, R.-R.; Jia, Y.-X. J. Am. Chem. Soc. 2018, 140, 13945. |
| [5] | (i) Zeidan, N.; Beisel, T.; Ross, R.; Lautens, M. Org. Lett. 2018, 20, 7332. |
| [5] | (j) Yang, P.; You, S.-L. Org. Lett. 2018, 20, 7684. |
| [6] | (a) Ma, D.; Cai, Q. Acc. Chem. Res. 2008, 41, 1450. |
| [6] | (b) Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359. |
| [7] | (a) Gao, D.; Parvez, M.; Back, T. G. Chem. Eur. J. 2010, 16, 14281. |
| [7] | (b) Bernini, R.; Cacchi, S.; Fabrizi, G.; Filisti, E.; Sferrazza, A. Synlett 2009, 1480. |
| [7] | (c) Yan, S.; Wu, H.; Wu, N.; Jiang, Y. Synlett 2007, 2699. |
| [7] | (d) Osuka, A.; Mori, Y.; Suzuki, H. Chem. Lett. 1982, 12, 2031. |
| [8] | Fortanet, J. G.; Kessler, F.; Buchwald, S. L. J. Am. Chem. Soc. 2009, 131, 6676. |
| [9] | (a) Liang, R.-X.; Zhong, C.; Liu, Z.-H.; Yang, M.; Tang, H.-W.; Chen, J.-F.; Yang, Y.-F.; Jia, Y.-X. ACS Catal. 2021, 11, 1827. |
| [9] | (b) Xu, H.-B.; Zhu, Y.-Y.; Dong, L. J. Org. Chem. 2019, 84, 16286. |
| [9] | (c) Jana, N.; Zhou, F.; Driver, T. G. J. Am. Chem. Soc. 2015, 137, 6738. |
| [9] | (d) Kong, C.; Driver, T. G. Org. Lett. 2015, 17, 802. |
/
| 〈 |
|
〉 |