

Chinese Journal of Organic Chemistry >
Recent Advances in Hydrochlorination of Alkenes
Received date: 2021-05-31
Revised date: 2021-07-15
Online published: 2021-08-19
Organochlorides have been widely used in medicine, pesticides, materials and other fields. In addition, organochlorides are also important synthetic building blocks. They have participated in a variety of reactions, such as free radical reactions, substitution reactions and cross-coupling reactions. The hydrochlorination of olefins is one of the most direct and efficient methods for the synthesis of organochlorides, and remakable breakthroughs have been made in the past thirty years. The research progress of olefin hydrochlorination in the past three decades is introduced. According to whether metal catalysis is involved, it is mainly classified into two categories: metal-free alkene hydrochlorination and metal-catalyzed alkene hydrochlorination. Different type of hydrochlorination reactions and related mechanisms are summarized. In addition, the future development direction of alkene hydrochlorination is prospected.
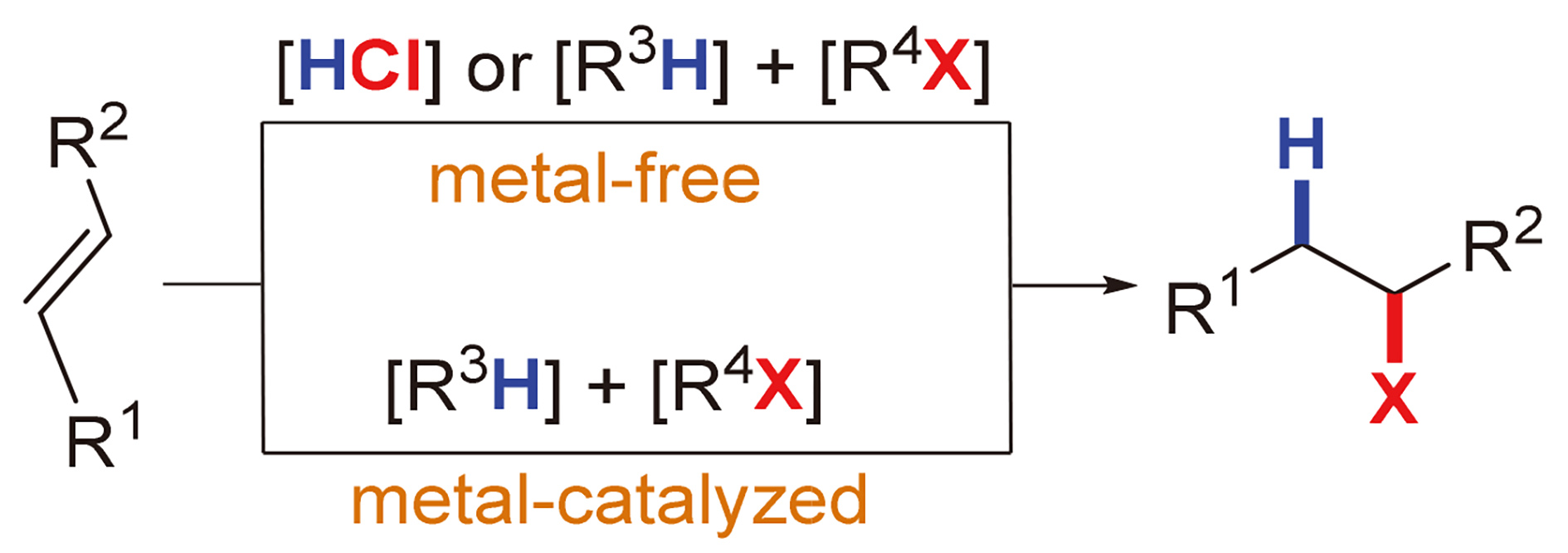
Key words: alkene; hydrochlorination; hydrogen chloride; organochloride; metal catalysis
Yaoxin Wang , Chen Cui , Xiaohui Yang . Recent Advances in Hydrochlorination of Alkenes[J]. Chinese Journal of Organic Chemistry, 2021 , 41(10) : 3808 -3815 . DOI: 10.6023/cjoc202105057
| [1] | (a) Kyne, S. H.; Lefèvre, G.; Ollivier, C.; Petit, M.; Cladera, C. A. R.; Fensterbank, L. Chem. Soc. Rev. 2020, 49, 8501. |
| [1] | (b) Liu, Q.; Zhang, L.; Mo, F. Acta Chim. Sinica 2020, 78, 1297. (in Chinese) |
| [1] | (刘谦益, 张雷, 莫凡洋, 化学学报, 2020, 78, 1297.) |
| [1] | (c) Togo, H. Advanced Free Radical Reactions for Organic Synthesis, Elsevier, Amsterdam, 2004. |
| [2] | Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry, 5th ed., Springer, New York, 2007. |
| [3] | For reviews, see: (a) Cheng, L.; Zhou, Q.-L. Acta Chim. Sinica 2020, 78, 1017. (in Chinese) |
| [3] | (程磊, 周其林, 化学学报, 2020, 78, 1017.) |
| [3] | (b) Zweig, J. E.; Kim, D. E.; Newhouse, T. R. Chem. Rev. 2017, 117, 11680. |
| [3] | (c) Liu, N.-W.; Liang, S.; Manolikakes, G. Synthesis 2016, 48, 1939. |
| [3] | (d) Terao, J.; Kambe, N. Acc. Chem. Res. 2008, 41, 1545. |
| [3] | (e) Rudolph, A.; Lautens, M. Angew. Chem., Int. Ed. 2009, 48, 2656. |
| [3] | For select papers, see: (f) Yao, D.; Zhang, J.; Xu, L. Chin. J. Org. Chem. 2020, 40, 1673. (in Chinese) |
| [3] | (姚丹丹, 张金利, 徐亮, 有机化学, 2020, 40, 1673.) |
| [3] | (g) Ma, D.; Niu, S.; Zhao, J.; Jiang, X.; Jiang, Y.; Zhang, X.; Sun, T. Chin. J. Chem. 2017, 35, 1661. |
| [3] | (h) Wang, X.; Wang, S. L.; Xue, W. C.; Gong, H. G. J. Am. Chem. Soc. 2015, 137, 11562. |
| [3] | (i) Gong, T.; Jiang, Y.; Fu, Y. Chin. Chem. Lett. 2014, 25, 397. |
| [3] | (j) Atack, T. C.; Cook, S. P. J. Am. Chem. Soc. 2012, 138, 6139. |
| [3] | (k) Dudnik, A. S.; Fu, G. C. J. Am. Chem. Soc. 2012, 134, 10693. |
| [4] | (a) Kohlmeyer, C.; Schafer, A.; Huy, P. H.; Hilt, G. ACS Catal. 2020, 10, 11567. |
| [4] | (b) Mohite, A. R.; Phatake, R. S.; Dubey, P.; Agbaria, M.; Shames, A. I.; Lemcoff, N. G.; Reany, O. J. Org. Chem. 2020, 85, 12901. |
| [4] | (c) Zheng, D.; Zhou, A.; Zhu, X.; Zheng, H.; Sun, X. Chin. J. Org. Chem. 2016, 36, 137. (in Chinese) |
| [4] | (郑大贵, 周安西, 祝显虹, 郑洪富, 孙向前, 有机化学, 2016, 36, 137.) |
| [4] | (d) Huy, P. H.; Motsch, S.; Kappler, S. M. Angew. Chem., Int. Ed. 2016, 55, 10145. |
| [4] | (e) Vanos, C. M.; Lambert, T. H. Angew. Chem., Int. Ed. 2011, 50, 12222. |
| [5] | King, S. M.; Ma, X.; Herzon, S. B. J. Am. Chem. Soc. 2014, 136, 6884. |
| [6] | (a) Yu, P.; Bismuto, A.; Morandi, B. Angew. Chem., Int. Ed. 2020, 59, 2904. |
| [6] | (b) Zeng, X.; Liu, S.; Hammond, G. B.; Xu, B. ACS Catal. 2018, 8, 904. |
| [6] | (c) Derosa, J.; Cantu, A. L.; Boulous, M. N.; O'Duill, M. L.; Turnbull, J. L.; Liu, Z.; De La Torre, D. M.; Engle, K. M. J. Am. Chem. Soc. 2017, 139, 5183. |
| [6] | (d) Zeng, X.; Lu, Z.; Liu, S.; Hammond, G. B.; Xu, B. J. Org. Chem. 2017, 82, 13179. |
| [6] | (e) Xu, C.; Ma, C.; Xiao, F.; Chen, H. Chin. Chem. Lett. 2016, 27, 1683. |
| [7] | For select reviews, see: (a) Guillemard, L.; Kaplaneris, N.; Ackermann, L.; Johansson, M. J. Nat. Rev. Chem. 2021, 5, 522. |
| [7] | (b) Petrone, D. A.; Ye, J.; Lautens, M. Chem. Rev. 2016, 116, 8003. |
| [7] | (c) Liu, W.; Groves, J. T. Acc. Chem. Res. 2015, 48, 1727. |
| [7] | For select papers, see: (d) Fawcett, A.; Keller, M. J.; Herrera, Z.; Hartwig, J. F. Angew. Chem., Int. Ed. 2021, 60, 8276. |
| [7] | (e) Herron, A. N.; Liu, D.; Xia, G.; Yu, J.-Q. J. Am. Chem. Soc. 2020, 142, 2766. |
| [7] | (f) Zhu, Y.; Shi, J.; Yu, W. Org. Lett. 2020, 22, 8899. |
| [7] | (g) Li, G.; Dilger, A. K.; Cheng, P. T.; Ewing, W. R.; Groves, J. T. Angew. Chem., Int. Ed. 2018, 57, 1251. |
| [7] | (h) Quinn, R. K.; Kçnst, Z. A.; Michalak, S. E.; Schmidt, Y.; Szklarski, A. R.; Flores, A. R.; Nam, S.; Horne, D. A.; Vanderwal, C. D.; Alexanian, E. J. J. Am. Chem. Soc. 2016, 138, 696. |
| [8] | Smith, M. B.; March, J. March's Advanced Organic Chemistry, John Wiley and Sons, New York, 2001. |
| [9] | (a) Fahey, R. C.; McPherson, C. A. J. Am. Chem. Soc. 1971, 93, 2445. |
| [9] | (b) Stille, J. K.; Sonnenberg, F. M.; Kinstle, T. H. J. Am. Chem. Soc. 1966, 88, 4922. |
| [9] | (c) Brown, H. C.; Rei, M.-H. J. Org. Chem. 1966, 31, 1090. |
| [9] | (d) Dewar, M. J. S.; Fahey, R. C. J. Am. Chem. Soc. 1963, 85, 2245. |
| [9] | (e) Ecke, G. G.; Cook, N. C.; Whitmore, F. C. J. Am. Chem. Soc. 1950, 72, 1511. |
| [9] | (f) Schmerling, L. J. Am. Chem. Soc. 1946, 68, 195. |
| [9] | (g) Whitmore, F. C.; Johnston, F. J. Am. Chem. Soc. 1933, 55, 5020. |
| [10] | Onitsuka, S.; Jin, Y. Z.; Shaikh, A. C.; Furuno, H.; Inanaga, J. Molecules 2012, 17, 11469. |
| [11] | Jin, Y. Z.; Yasuda, N.; Furuno, H.; Inanaga, J. Tetrahedron Lett. 2003, 44, 8765. |
| [12] | Ballini, R.; Bosica, G.; Parrini, M. Tetrahedron Lett. 1998, 39, 7963. |
| [13] | (a) Kropp, P. J.; Daus, K. A.; Tubergen, M. W.; Kepler, K. D.; Wil-son, V. P.; Craig, S. L.; Baillargeon, M. M.; Breton, G. W. J. Am. Chem. Soc. 1993, 115, 3071. |
| [13] | (b) Kropp, P. J.; Daus, K. A.; Crawford, S. D.; Tubergen, M. W.; Kepler, K. D.; Craig, S. L.; Wilson, V. P. J. Am. Chem. Soc. 1990, 112, 7433. |
| [14] | de Mattos, M. C. S.; Sanseverino, A. M. Synth. Commun. 2001, 30, 1975. |
| [15] | Tanemura, K. Tetrahedron Lett. 2018, 59, 4293. |
| [16] | Boudjouk, P.; Kim, B. K.; Han, B. H. Synth. Commun. 1996, 26, 3479. |
| [17] | Yadav, V. K.; Babu, K. G. Eur. J. Org. Chem. 2005, 2005, 452. |
| [18] | Liang, S.; Hammond, G. B.; Xu, B. Green. Chem. 2018, 20, 680. |
| [19] | Schevenels, F. T.; Shen, M.; Snyder, S. A. J. Am. Chem. Soc. 2017, 139, 6329. |
| [20] | Wilger, D. J.; Grandjean, J. M. M.; Lamment, T. R.; Nicewicz, D. A. Nat. Chem. 2014, 6, 720. |
| [21] | Alper, H.; Huang, Y. Organometallics 1991, 10, 1665. |
| [22] | Fahey, R. C.; Monahan, M. W.; Mcphersons, C. A. J. Am. Chem. Soc. 1970, 92, 2816. |
| [23] | Podhajsky, S. M.; Sigman, M. S. Organometallics. 2007, 26, 5680. |
| [24] | Gaspar, B.; Carreira, E. M. Angew. Chem., Int. Ed. 2008, 47, 5758. |
| [25] | Waser, J.; Gaspar, B.; Nambu, H.; Carreira, E. M. J. Am. Chem. Soc. 2006, 128, 11693. |
/
| 〈 |
|
〉 |