

Chinese Journal of Organic Chemistry >
Sc(OTf)3-Catalyzed Reaction of Purines with o-Hydroxybenzyl Alcohols for Construction of Acyclic Nucleosides
Received date: 2021-06-05
Revised date: 2021-07-16
Online published: 2021-08-19
Supported by
National Natural Science Foundation of China(22071046); Zhongyuan Scholar(212101510004); National Government Guides Local Special Funds for the Development of Science and Technology(YDZX20204100001786)
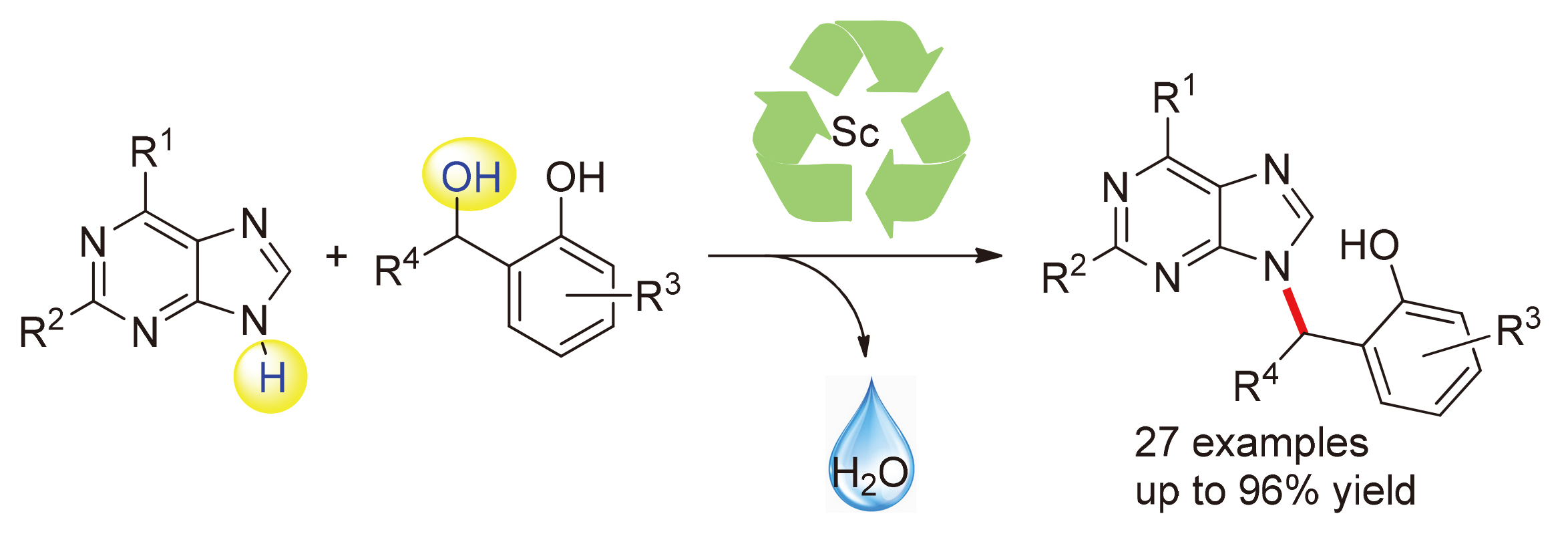
Efficient alkylation of purines with ortho-hydroxybenzyl alcohols under mild condition has been achieved by Sc(OTf)3 catalysis. This C—N bond formation process is proposed to proceed through an ortho-quinone methide intermediate. The reaction allows for efficient synthesis of various acyclic nucleoside analogs, with excellent yields of up to 96%, across a broad range of substrates and the yields were maintained as the reactions were scaled up.
Chao Xia , Dongchao Wang , Haiming Guo . Sc(OTf)3-Catalyzed Reaction of Purines with o-Hydroxybenzyl Alcohols for Construction of Acyclic Nucleosides[J]. Chinese Journal of Organic Chemistry, 2021 , 41(11) : 4391 -4399 . DOI: 10.6023/cjoc202106010
| [1] | (a) Willis, N. J.; Bray, C. D. Chem.-Eur. J. 2012, 18, 9160. |
| [1] | (b) Bai, W.-J.; David, J. G.; Feng, Z.-G.; Weaver, M. G.; Wu, K.-L.; Pettus, T. R. R. Acc. Chem. Res. 2014, 47, 3655. |
| [1] | (c) Jaworski, A. A.; Scheidt, K. A. J. Org. Chem. 2016, 81, 10145. |
| [1] | (d) Yang, B.; Gao, S. Chem. Soc. Rev. 2018, 47, 7926. |
| [2] | (a) Meisinger, N.; Roiser, L.; Monkowius, U.; Himmelsbach, M.; Robiette, R.; Waser, M. Chem.-Eur. J. 2017, 23, 5137. |
| [2] | (b) Suneja, A.; Schneider, C. Org. Lett. 2018, 20, 7576. |
| [2] | (c) Pandit, R. P.; Kim, S. T.; Ryu, D. H. Angew. Chem., Int. Ed. 2019, 58, 13427. |
| [3] | (a) Bu, H.-Z.; Li, H.-H.; Luo, W.-F.; Luo, C.; Qian, P.-C.; Ye, L.-W. Org. Lett. 2020, 22, 648. |
| [3] | (b) Feng, S.; Yang, B.; Chen, T.; Wang, R.; Deng, Y.-H.; Shao, Z. J. Org. Chem. 2020, 85, 5231. |
| [3] | (c) Huang, H.-M.; Wu, X.-Y.; Leng, B.-R.; Zhu, Y.-L.; Meng, X.-C.; Hong, Y.; Jiang, B.; Wang, D.-C. Org. Chem. Front. 2020, 7, 414. |
| [3] | (d) Shi, H.; Wang, L.; Li, S.-S.; Liu, Y.; Xu, L. Org. Chem. Front. 2020, 7, 747. |
| [3] | (e) Tanaka, K.; Asada, Y.; Hoshino, Y.; Honda, K. Org. Biomol. Chem. 2020, 18, 8074. |
| [3] | (f) Tu, M.-S.; Liu, S.-J.; Zhong, C.; Zhang, S.; Zhang, H.; Zheng, Y.-L.; Shi, F. J. Org. Chem. 2020, 85, 5403. |
| [3] | (g) You, Y.; Li, T.-T.; Yuan, S.-P.; Xie, K.-X.; Wang, Z.-H.; Zhao, J.-Q.; Zhou, M.-Q.; Yuan, W.-C. Chem. Commun. 2020, 56, 439. |
| [4] | (a) Mei, G.-J.; Zhu, Z.-Q.; Zhao, J.-J.; Bian, C.-Y.; Chen, J.; Chen, R.-W.; Shi, F. Chem. Commun. 2017, 53, 2768. |
| [4] | (b) Lam, H.; Qureshi, Z.; Wegmann, M.; Lautens, M. Angew. Chem., Int. Ed. 2018, 57, 16185. |
| [4] | (c) Sun, M.; Ma, C.; Zhou, S.-J.; Lou, S.-F.; Xiao, J.; Jiao, Y.; Shi, F. Angew. Chem., Int. Ed. 2019, 58, 8703. |
| [4] | (d) Suneja, A.; Loui, H. J.; Schneider, C. Angew. Chem., Int. Ed. 2020, 59, 5536. |
| [4] | (e) Yan, R.-J.; Liu, B.-X.; Xiao, B.-X.; Du, W.; Chen, Y.-C. Org. Lett. 2020, 22, 4240. |
| [4] | (f) Zhou, S.-J.; Sun, M.; Wang, J.-Y.; Yu, X.-Y.; Lu, H.; Zhang, Y.-C.; Shi, F. Eur. J. Org. Chem. 2020, 4301. |
| [5] | Xia, C.; Wang, D.-C.; Qu, G.-R.; Guo, H.-M. Org. Chem. Front. 2020, 7, 1474. |
| [6] | (a) Wang, Y.; Zhang, C.; Wang, H.; Jiang, Y.; Du, X.; Xu, D. Adv. Synth. Catal. 2017, 359, 791. |
| [6] | (b) Wu, J.-L.; Wang, J.-Y.; Wu, P.; Mei, G.-J.; Shi, F. Org. Chem. Front. 2017, 4, 2465. |
| [6] | (c) Zhou, J.; Huang, W.-J.; Jiang, G.-F. Org. Lett. 2018, 20, 1158. |
| [6] | (d) Wu, Q.; Li, G.-L.; Yang, S.; Shi, X.-Q.; Huang, T.-Z.; Du, X.-H.; Chen, Y. Org. Biomol. Chem. 2019, 17, 3462. |
| [6] | (e) Chu, M.-M.; Chen, X.-Y.; Wang, Y.-F.; Qi, S.-S.; Jiang, Z.-H.; Xu, D.-Q.; Xu, Z.-Y. J. Org. Chem. 2020, 85, 9491. |
| [6] | (f) Zhang, Y.-Z.; Sheng, F.-T.; Zhu, Z.; Li, Z.-M.; Zhang, S.; Tan, W.; Shi, F. Org. Biomol. Chem. 2020, 18, 5688. |
| [7] | Lai, Z.; Wang, Z.; Sun, J. Org. Lett. 2015, 17, 6058. |
| [8] | (a) Guo, W.; Wu, B.; Zhou, X.; Chen, P.; Wang, X.; Zhou, Y.-G.; Liu, Y.; Li, C. Angew. Chem., Int. Ed. 2015, 54, 4522. |
| [8] | (b) Lai, Z.; Sun, J. Synlett 2016, 27, 555. |
| [8] | (c) Roy, D.; Panda, G. Tetrahedron 2018, 74, 6270. |
| [9] | (a) Huang, H.; Kang, J. Y. Org. Lett. 2017, 19, 5988. |
| [9] | (b) Gu, X.; Yuan, H.; Jiang, J.; Wu, Y.; Bai, W.-J. Org. Lett. 2018, 20, 7229. |
| [10] | (a) Chen, M.; Han, Y.; Ma, D.; Wang, Y.; Lai, Z.; Sun, J. Chin. J. Chem. 2018, 36, 587. |
| [10] | (b) Pelayo, S. G.; López, L. A. Eur. J. Org. Chem. 2017, 2017, 6003. |
| [10] | (c) Chen, L.-M.; Zhao, J.; Xia, A.-J.; Guo, X.-Q.; Gan, Y.; Zhou, C.; Yang, Z.-J.; Yang, J.; Kang, T.-R. Org. Biomol. Chem. 2019, 17, 8561. |
| [10] | (d) Osyanin, V. A.; Osipov, D. V.; Nakushnov, V. Y.; Zemtsova, M. N.; Klimochkin, Y. N. Chem. Heterocycl. Compd. 2015, 51, 984. |
| [11] | (a) Kelley, J. L.; Kelsey, J. E.; Hall, W. R.; Krochmal, M. P.; Schaeffer, H. J. J. Med. Chem. 1981, 24, 753. |
| [11] | (b) Vandenriessche, F.; Snoeck, R.; Janssen, G.; Hoogmartens, J.; Aerschot, A. V.; Clercq, E. D.; Herdewijn, P. J. Med. Chem. 1992, 35, 1458. |
| [11] | (c) Sekiyama, T.; Hatsuya, S.; Tanaka, Y.; Uchiyama, M.; Ono, N.; Iwayama, S.; Oikawa, M.; Suzuki, K.; Okunishi, M.; Tsuji, T. J. Med. Chem. 1998, 41, 1284. |
| [11] | (d) Hakimelahi, G. H.; Ly, T. W.; Movahedi, A. A. M.; Jain, M. L.; Zakerinia, M.; Davari, H.; Mei, H.-C.; Sambaiah, T.; Moshfegh, A. A.; Hakimelahi, S. J. Med. Chem. 2001, 44, 3710. |
| [11] | (e) Hernández, A.-I.; Balzarini, J.; Karlsson, A.; Camarasa, M.-J.; Pérez, M.-J. J. Med. Chem. 2002, 45, 4254. |
| [11] | (f) Clercq, E. D. J. Med. Chem. 2019, 62, 7322. |
| [12] | (a) Palazzolo, M. A.; Nigro, M. J.; Iribarren, A. M.; Lewkowicz, E. S. Eur. J. Org. Chem. 2016, 2016, 921. |
| [12] | (b) Derudas, M.; Vanpouille, C.; Carta, D.; Zicari, S.; Andrei, G.; Snoeck, R.; Brancale, A.; Margolis, L.; Balzarini, J.; McGuigan, C. J. Med. Chem. 2017, 60, 7876. |
| [12] | (c) Lee, S.-J.; Ahn, J.-G.; Seo, J.; Ha, H.-J.; Cho, C.-W. Org. Biomol. Chem. 2018, 16, 9477. |
| [12] | (d) Zhang, H.; Xie, M.; Qu, G.; Chang, J. Org. Lett. 2019, 21, 120. |
| [12] | (e) Baddi, L.; Ouzebla, D.; Mansouri, A.-E.; Smietana, M.; Vasseur, J.-J.; Lazrek, H. B. Nucleosides, Nucleotides Nucleic Acids 2021, 40, 43. |
| [13] | (a) Sun, H.-L.; Chen, F.; Xie, M.-S.; Guo, H.-M.; Qu, G.-R.; He, Y.-M.; Fan, Q.-H. Org. Lett. 2016, 18, 2260. |
| [13] | (b) Zhou, P.; Xie, M.-S.; Qu, G.-R.; Li, R.-L.; Guo, H.-M. Asian J. Org. Chem. 2016, 5, 1100. |
| [13] | (c) Liang, L.; Xie, M.-S.; Qin, T.; Zhu, M.; Qu, G.-R.; Guo, H.-M. Org. Lett. 2017, 19, 5212. |
| [13] | (d) Wang, H.-X.; Yu, L.-L.; Xie, M.-S.; Wu, J.; Qu, G-R.; Ding, K.-L.; Guo, H.-M. Chem.-Eur. J. 2018, 24, 1425. |
| [13] | (e) Liang, T.; Xie, M.-S.; Qu, G.-R.; Guo, H.-M. Asian J. Org. Chem. 2019, 8, 1405. |
| [13] | (f) Hao, E.-J.; Li, G.-X.; Liang, Y.-R.; Xie, M.-S.; Wang, D.-C.; Jiang, X.-H.; Cheng, J.-Y.; Shi, Z.-X.; Wang, Y.; Guo, H.-M. J. Med. Chem. 2021, 64, 2077. |
| [13] | (g) Xia, C.; Wang, D.-C.; Qu, G.-R.; Guo, H.-M. Org. Chem. Front. 2021, 8, 2569. |
/
| 〈 |
|
〉 |