

Chinese Journal of Organic Chemistry >
Samarium Diiodide Promoted the Addition-Ring-Opening Reaction of 2-Piperidinone with α,β-Unsaturated Esters
Received date: 2021-06-22
Revised date: 2021-07-20
Online published: 2021-08-25
Supported by
Postgraduate Students Innovation Program(20KY0431)
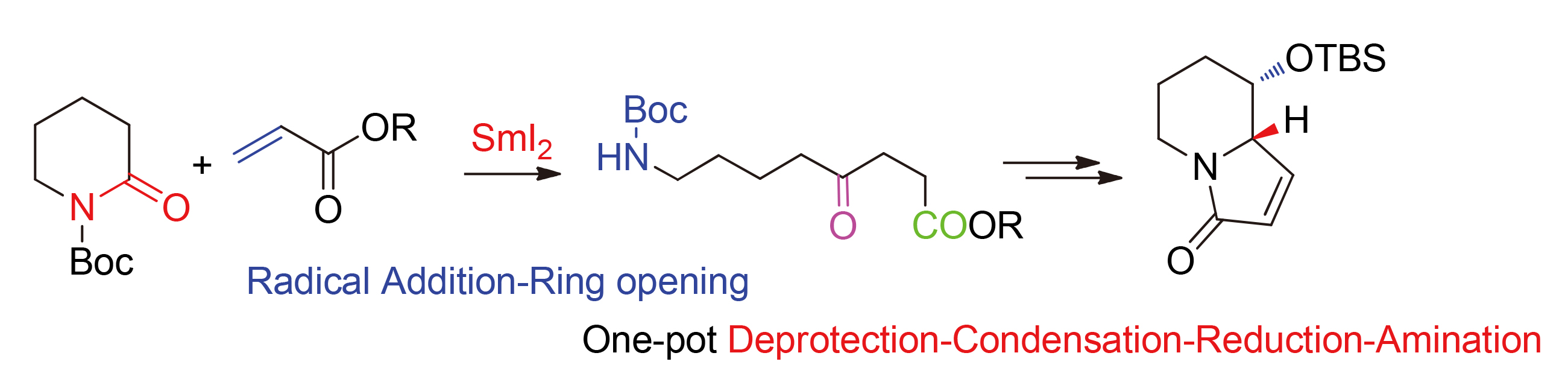
A convenient approach to N-Boc amino ketones 3a~3l has been developed, which features a SmI2 prompted one-pot radical addition-ring opening process of 2-piperidinone with α,β-unsaturated esters. Moreover, the indolizidine skeleton (6) has been successfully synthesized from the key methyl (S)-8-((tert-butoxycarbonyl)amino)-5-((tert-butyl- dimethylsilyl)oxy)-4-oxooctanoate (3e), which undergoes one-pot deprotection-condensation-reduction-amination process.
Jian-Ting Sun , Ling-Yan Chen , Bang-Guo Wei . Samarium Diiodide Promoted the Addition-Ring-Opening Reaction of 2-Piperidinone with α,β-Unsaturated Esters[J]. Chinese Journal of Organic Chemistry, 2021 , 41(11) : 4320 -4326 . DOI: 10.6023/cjoc202106040
| [1] | (a) Takahata, H.; Momose, T. The Alkaloids: Chemistry and Pharmacology, Elsevier, Toyama, 1993, pp. 189-256. |
| [1] | (b) Michael, J. P. Nat. Prod. Rep. 2007, 24, 191. |
| [2] | Asano, N.; Nash, R. J.; Molyneux, R. J.; Fleet, G. W. J. Tetrahedron: Asymmetry 2000, 11, 1645. |
| [3] | Zhang, J.; Morris-Natschke, S. L.; Ma, D.; Shang, X. F.; Yang, C. J.; Liu, Y. Q.; Lee, K. H. Med. Res. Rev. 2021, 41, 928. |
| [4] | (a) Guengerich, F. P.; Dimari, S. J.; Broquist, H. P. J. Am. Chem. Soc. 1973, 95, 2055. |
| [4] | (b) Kino, T.; Inamura, N.; Nakahara, K.; Kiyoto, S.; Goto, T.; Terano, H.; Kohsaka, M.; Aoki, H.; Imanaka, H. J. Antibiot. 1985, 38, 936. |
| [4] | (c) Yu, X.; Zhao, Y.; Wang, L.; Chen, X.; Su, Z.; Zhang, H.; Yuan, Q.; Wang, S. Biomed. Pharmacother. 2016, 84, 1654. |
| [5] | (a) Hohenschutz, L. D.; ArthurBell, E.; Jewess, P. J.; Leworthy, D. P.; Pryce, R. J.; Arnold, E.; Clardy, J. Phytochemistry 1981, 20, 811. |
| [5] | (b) Nash, R. J.; Fellows, L. E.; Dring, J. V.; Stirton, C. H.; Carter, D.; Hegarty, M. P.; Bell, E. A. Phytochemistry 1988, 27, 1403. |
| [6] | Whitby, K.; Pierson, T. C.; Geiss, B.; Lane, K.; Diamond, M. S. J. Virol. 2005, 79, 8698. |
| [7] | Huang, X.; Gao, S.; Fan, L. H.; Yu, S. S.; Liang, X. T. Planta Med. 2004, 70, 441. |
| [8] | Dhiman, M.; Parab, R. R.; Manju, S. L.; Desai, D. C.; Mahajan, G. B. Nat. Prod. Commun. 2012, 7, 1171. |
| [9] | Girard, P.; Namy, J. L.; Kagan, H. B. J. Am. Chem. Soc. 1980, 102, 2693. |
| [10] | For the selected SmI2 activated nitrone: (a) Rehák, J.; Fišera, L.; Kožíšek, J.; Bellovičová, L. Tetrahedron 2011, 67, 5762. |
| [10] | (b) Wu, S.-F.; Zheng, X.; Ruan, Y. P.; Huang, P. Q. Org. Biomol. Chem. 2009, 7, 2967. |
| [10] | (c) Wu, S. F.; Ruan, Y. P.; Zheng, X.; Huang, P. Q. Tetrahedron 2010, 66, 1653. |
| [10] | (d) Zhang, H. K.; Xu, S. Q.; Zhuang, J. J.; Ye, J. L.; Huang, P. Q. Tetrahedron 2012, 68, 6656. |
| [11] | For the selected SmI2 activated aza-hemiacetal: (a) Liu, X. K.; Qiu, S.; Xiang, Y. G.; Ruan, Y. P.; Zheng, X.; Huang, P. Q. J. Org. Chem. 2011, 76, 4952. |
| [11] | (b) Hu, K. Z.; Ma, J.; Qiu, S.; Zheng, X.; Huang, P. Q. J. Org. Chem. 2013, 78, 1790. |
| [11] | (c) Yoda, H.; Ujihara, Y.; Takabe, K. Tetrahedron Lett. 2001, 42, 9225. |
| [11] | (d) Yoda, H.; Kohata, N.; Takabe, K. Synth. Commun. 2003, 33, 1087. |
| [11] | (e) Zheng, X.; Feng, C. G.; Ye, J. L.; Huang, P. Q. Org. Lett. 2005, 7, 553. |
| [12] | For the selected SmI2 activated amide: (a) McDonald, C. E.; Galka, A. M.; Green, A. I.; Keane, J. M.; Kowalchick, J. E.; Micklitsch, C. M.; Wisnoski, D. D. Tetrahedron Lett. 2001, 42, 163. |
| [12] | (b) Martin, S. F.; Yang, C. P.; Laswell, W. L.; Rueeger, H. Tetrahedron Lett. 1988, 29, 6685. |
| [12] | (c) Yu, H.; Gai, T.; Sun, W. L.; Zhang, M. S. Chin. Chem. Lett. 2011, 22, 379. |
| [12] | (d) Huang, H.-M.; Procter, D. J. Angew. Chem., Int. Ed. 2017, 56, 14262. |
| [13] | For the selected SmI2 activated imide (a) Vacas, T.; Álvarez, E.; Chiara, J. L. Org. Lett. 2007, 9, 5445. |
| [13] | (b) Shi, S.; Szostak, M. Org. Lett. 2015, 17, 5144. |
| [13] | (c) Shi, S.; Szostak, M. Molecules 2017, 22, 2018. |
| [13] | (d) Ha, D. C.; Yun, C. S.; Lee, Y. J. Org. Chem. 2000, 65, 621. |
| [13] | (e) Farcas, S.; Namy, J.-L. Tetrahedron Lett. 2000, 41, 7299. |
| [13] | (f) Jensen, C. M.; Lindsay, K. B.; Taaning, R. H.; Karaffa, J.; Hansen, A. M.; Skrydstrup, T. J. Am. Chem. Soc. 2005, 127, 6544. |
| [13] | (g) Hansen, A. M.; Lindsay, K. B.; Sudhadevi Antharjanam, P. K.; Karaffa, J.; Daasbjerg, K.; Flowers, R. A.; Skrydstrup, T. J. Am. Chem. Soc. 2006, 128, 9616. |
| [14] | (a) Liu, R.-C.; Wei, J.-H.; Wei, B.-G.; Lin, G.-Q. Tetrahedron: Asymmetry 2008, 19, 2731. |
| [14] | (b) Wei, B.-G.; Chen, J.; Huang, P.-Q. Tetrahedron 2006, 62, 190. |
| [14] | (c) Nie, X.-D.; Mao, Z.-Y.; Zhou, W.; Si, C.-M.; Wei, B.-G.; Lin, G.-Q. Org. Chem. Front. 2020, 7, 76. |
| [14] | (d) Han, X.-L.; Nie, X.-D.; Feng, Y.-M.; Wei, B.-G.; Si, C.-M.; Lin, G. -Q. Chin. Chem. Lett. 2021, doi: 10.1016/j.cclet.2021.05.003. |
| [15] | (a) Han, P.; Mao, Z.-Y.; Si, C.-M.; Zhou, Z.; Wei, B.-G.; Lin, G.-Q. J. Org. Chem. 2019, 84, 914. |
| [15] | (b) Huang, P. Q.; Chen, G.; Zheng, X. J. Heterocycl. Chem. 2007, 44, 499. |
| [15] | (c) Si, C. M.; Huang, W.; Du, Z. T.; Wei, B. G.; Lin, G. Q. Org. Lett. 2014, 16, 4328. |
| [16] | Kwon, H. Y.; Park, C. M.; Lee, S. B.; Youn, J. H.; Kang, S. H. Chem.-Eur. J. 2008, 14, 1023. |
| [17] | Si, C.-M.; Mao, Z.-Y.; Dong, H. Q.; Du, Z. T.; Wei, B.-G.; Lin, G.-Q. J. Org. Chem. 2015, 80, 5824. |
| [18] | CCDC 2090177 (5) contains the supplementary crystallographic data for this paper. These data are free of charge from The Cambridge Crystallographic Centre via www.ccdc.cam.ac.uk/datarequest/cif. |
/
| 〈 |
|
〉 |