

Chinese Journal of Organic Chemistry >
Metal-Free Selenizative spiro-Tricyclization of N-Hydroxylethyl-N-arylpropiolamides
Received date: 2021-06-24
Revised date: 2021-08-22
Online published: 2021-09-03
Supported by
National Natural Science Foundation of China(21772067); National Natural Science Foundation of China(21762018); National Natural Science Foundation of China(21961014); Natural Science Foundation of Jiangxi Province(20192BCBL23009); Natural Science Foundation of Jiangxi Province(20202BABL203005); Youth Jinggang Scholars Program in Jiangxi Province
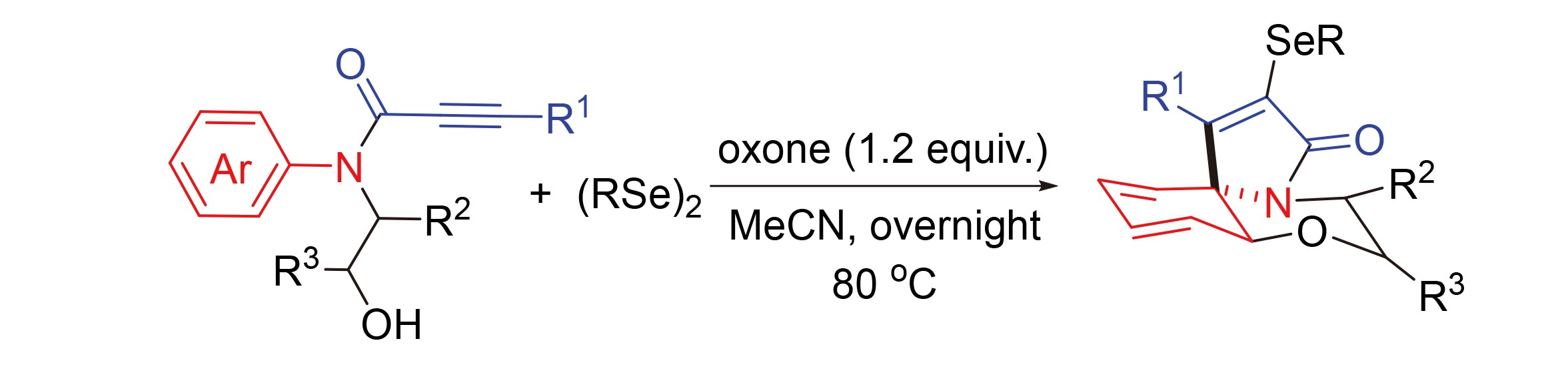
Facile ipso-cyclization and regioselective ortho-capture of N-hydroxylethyl-N-arylpropiolamides are reported for the synthesis of various selenium-containing benzo[b]pyrrolo[2,1-c][1,4]oxazin-3-ones. The reaction works well with high efficiency and broad reaction scope. In the process, it is believed that α-addition of the propiolamide, sequential ipso-cyclization of the aniline, and regioselective ortho-capture with a hydroxyl group are involved.
Yuchao Wang , Jinbiao Liu , Guanyinsheng Qiu , Yu Yang , Hongwei Zhou . Metal-Free Selenizative spiro-Tricyclization of N-Hydroxylethyl-N-arylpropiolamides[J]. Chinese Journal of Organic Chemistry, 2021 , 41(12) : 4798 -4807 . DOI: 10.6023/cjoc202106044
| [1] | (a) Wang, Z. Org. Chem. Front. 2020, 7, 3815. |
| [1] | (b) Lanke, V.; Marek, I. Chem. Sci. 2020, 11, 9378. |
| [1] | (c) Li, C. X.; Ragab, S. S.; Liu, G. D.; Tang, W. J. Nat. Prod. Rep. 2020, 37, 276. |
| [1] | (d) Quiclet-Sire, B.; Zard, S. Z. Sci. China: Chem. 2019, 62, 1450. |
| [1] | (e) Xu, P. W.; Yu, J. S.; Chen, C.; Cao, Z. Y.; Zhou, F.; Zhou, J. ACS Catal. 2019, 9, 1820. |
| [2] | For selected reviews, see: (a) Yang, W.-C.; Zhang, M.-M.; Feng, J.-G. Adv. Synth. Catal. 2020, 362, 4446. |
| [2] | (b) Huck, C. J.; Sarlah, D. Chem. 2020, 6, 1589. |
| [2] | (c) Xia, Z. L.; Xu-Xu, Q.-F.; Zheng, C.; You, S.-L. Chem. Soc. Rev. 2020, 49, 286. |
| [2] | (d) Lv, S. D.; Zhang, G. F.; Chen, J. B.; Gao, W. Adv. Synth. Catal. 2020, 362, 462. |
| [2] | (e) Zeidan, N.; Lautens, M. Synthesis 2019, 51, 4137. |
| [3] | For recent examples, see: (a) Grams, R. J.; Garcia, C. J.; Szwetkoski, C.; Santos, W. L. Org. Lett. 2020, 22, 7013. |
| [3] | (b) Lu, L.; Guo, C. G.; Peng, H.; Jiang, H.; Lei, M.; Yin, B. L. Org. Lett. 2019, 21, 2602. |
| [3] | (c) Lee, W.; Lee, Y.; Yoo, M.; Han, S.; Kim, H. Org. Chem. Front. 2020, 7, 3209. |
| [3] | (d) Song, L.; Tian, G.; Van, M. L.; Van der Eycken, E. V. Org. Lett. 2020, 22, 6537. |
| [3] | (e) Cheng, C.; Zuo, X.; Tu, D.; Wan, B.; Zhang, Y. Org. Lett, 2020, 22, 4985. |
| [3] | (f) Pramanik, M.; Choudhuri, K.; Chakrabory, S.; Ghosh, A.; Mal, P. Chem. Commun. 2020, 56, 2991. |
| [3] | (g) Zhou, M.-B.; Li, Y.; Ouyang, X.; Li, J.-H. Sci. China: Chem. 2020, 63, 222. |
| [4] | For selected reviews, see: (a) Song, R.; Xie, Y. Chin. J. Chem. 2017, 35, 280. |
| [4] | (b) Vessally, E.; Babazadeh, M.; Didehban, K.; Hosseinian, A.; Edjlali, L. Curr. Org. Chem. 2018, 22, 286. |
| [4] | (c) Reddy, C. R.; Prajapti, S. K.; Warudikar, K.; Ranjan, R.; Rao, B. B. Org. Biomol. Chem. 2017, 15, 3130. |
| [4] | (d) Ni, S.; Zhou, J.; Mei, H.; Han, J. Tetrahedron Lett. 2018, 59, 1309. |
| [5] | Nair, A. M.; Shinde, A. H.; Kumar, S.; Volla, C. M. R. Chem. Commun. 2020, 56, 12367. |
| [5] | (b) Reddy, C. R.; Dattahari, H.; Subbarao, M.; Aila, M.; Prajapti, S. K. Org. Lett. 2020, 22, 5342. |
| [5] | (c) Nair, A. M.; Halder, I.; Khan, S.; Volla, C. M. R. Adv. Synth. Catal. 2020, 362, 224. |
| [5] | (d) Liu, Y.; Wang, Q.-L.; Chen, Z.; Zhou, Q.; Xiong, B.-Q.; Zhang, P.-L.; Tang, K.-W. Chem. Commun. 2019, 55, 12212. |
| [6] | For selected recent examples, see: (a) Zhang, N.; Zuo, H.; Xu, C.; Pan, J.; Sun, J.; Guo, C. Chin. Chem. Lett. 2020, 31, 337. |
| [6] | (b) Chen, Y.; Chen, Y.-J.; Guan, Z.; He, Y.-Y. Tetrahedron 2019, 75, 130763. |
| [6] | (c) Wang, C.-S.; Roisnel, T. P.; Dixneuf, H.; Soule, J.-F. Adv. Synth. Catal. 2019, 361, 445. |
| [7] | Wang, Y.-C.; Liu, J.-B.; Zhou, H. W.; Xie, W.; Rojsittisak, P.; Qiu, G. J. Org. Chem. 2020, 85, 1906. |
| [8] | (a) Wang, Y.-C.; Wang, R.-X.; Qiu, G.; Zhou, H.; Xie, W.; Liu, J.-B. Org. Chem. Front. 2019, 6, 2471. |
| [8] | (b) Ren, S.-F.; Wang, Y.-C.; Liu, J.-B.; Qiu, G. Chin. J. Org. Chem. 2021, 41, 3652. (in Chinese) |
| [8] | ( 任尚峰, 王玉超, 刘晋彪, 邱观音生, 有机化学, 2021, 41, 3652.) |
| [8] | (c) Yuan, S.; Zhou, H.; Gao, L.; Liu, J.-B.; Qiu, G. Org. Lett. 2018, 20, 562. |
| [8] | (d) Qiu, G.; Li, Y.; Ma, L.; Zhou, H. Org. Chem. Front. 2017, 4, 1069. |
| [8] | (e) Lin, C.-K.; Zhang, C.-X.; Huang, K. K.; Wang, Y.-C.; Liu, J.-B. J. Jiangxi Univ. Sci. Technol. 2020, 41, 1. (in Chinese) |
| [8] | ( 林川凯, 张彩霞, 黄棵棵, 王玉超, 刘晋彪, 江西理工大学学报, 2020, 41, 1.) |
| [8] | (f) Rodrigues, I.; Barcellos, A. M.; Belladonaet, A. L.; Roehrs, J. A.; Cargnelutti, R.; Alves, D.; Perin, G.; Schumacher, R. F. Tetrahedron 2018, 74, 4242. |
| [8] | (g) Goulart, H. A.; Neto, J. S. S.; Barcellos, A. M.; Barcellos, T.; Silva, M. S.; Alves, D.; Jacob, R. G.; Lenardão, E. J.; Perin, G. Adv. Synth. Catal. 2019, 361, 3403. |
| [8] | (h) Wang, Y.-C.; Huang, K.; Lai, X.; Shi, Z.; Liu, J.; Qiu, G. Org. Biomol. Chem. 2021, 19, 1940. |
| [9] | (a) Zaman, M.; Hasan, M.; Pehkow, A. A.; Van Hecke, K.; Van der Eycken, E. V. Pereshivko, O. P.; Peshkow, V. A. Adv. Synth. Catal. 2020, 362, 261. |
| [9] | (b) Singh, K.; Malviya, B. K.; Jaiswal, P. K.; Verma, V. P.; Chimni, S. S.; Sharma, S. Org. Lett. 2019, 21, 6726. |
| [10] | Yugandhar, D.; Kuriakose, S.; Nanubolu, J. B.; Srivastava, A. K. Org. Lett. 2016, 18, 1040. |
| [11] | Reviews, see: (a) Ivaanova, A.; Arsenyan, P. Coord. Chem. Rev. 2018, 370, 55. |
| [11] | (b) Yu, L.; Wang, J.; Cao, H.; Ding, K.; Xu, Q. Chin. J. Org. Chem. 2014, 34, 1986. (in Chinese) |
| [11] | ( 俞磊, 王俊, 曹洪恩, 丁克鸿, 徐清, 有机化学, 2014, 34, 1986.) |
| [12] | For selected recent example, see: (a) Belladona, A. L.; Cervo, R.; Alves, D.; Barcellos, T.; Cargnelutti, R.; Schumacher, R. F. Tetrahedron Lett. 2020, 61, 152035. |
| [12] | (b) Hua, G.; Cordes, D. B.; Slawin, A. M. Z.; Woollins, J. D. ACS Omerga 2020, 5, 11737. |
| [13] | For selected recent example, see: (a) Zhang, Q.-B.; Yuan, P.-F.; Kai, L.-L.; Liu, K.; Ban, Y.-L.; Wang, X.-Y.; Wu, L.-Z.; Liu, Q. Org. Lett. 2019, 21, 885. |
| [13] | (b) Ding, C.; Yu, Y.; Yu, Q.; Xie, Z.; Zhou, Y.; Zhou, J.; Liang, G.; Song, Z. ChemCatChem 2018, 10, 5397. |
| [13] | (c) Liu, M.; Li, Y.; Yu, L.; Xu, Q.; Jiang, X. Sci. China: Chem. 2018, 61, 294. |
| [14] | (a) Chen, J.-M.; Qi, L.; Zhang, L.; Li, L.-J.; Hou, C.-Y.; Li, W.; Wang, L.-J. J. Org. Chem. 2020, 85, 10924. |
| [14] | (b) Leng, T.; Wu, G.; Zhou, Y.; Gao, W.; Ding, J.; Huang, X.; Liu, M.; Wu, H. Adv. Synth. Catal. 2018, 360, 4336. |
| [15] | Perin, G.; Soares, L. K.; Hellwig, P. S.; Silva, M. S.; Neto, J. S. S.; Roehrs, J. A.; Barcellos, T.; Lenardao, E. J. New J. Chem. 2019, 43, 6323. |
| [16] | Recchi, A. M. S.; Rosa, P. H. P.; Back, D. F.; Zeni, G. Org. Biomol. Chem. 2020, 18, 3544. |
| [17] | (a) Hua, J.; Fang, Z.; Bian, M.; Ma, T.; Yang, M.; Xu, J.; Liu, C.; He, W.; Zhu, N.; Yang, Z.; Guo, K. ChemSusChem 2020, 13, 2053. |
| [17] | (b) Sahoo, H.; Grandi, G. S.; Ramakrishna, I.; Baiya, M. Org. Biomol. Chem. 2019, 17, 10163. |
| [17] | (c) Zhang, R.; Xu, P.; Wang, S.-Y.; Ji, S.-J. J. Org. Chem. 2019, 84, 12324. |
/
| 〈 |
|
〉 |