

Chinese Journal of Organic Chemistry >
Solvent Mediated Selective C—H Bond Iodination of Pyrrolo[1,2-a]quinoxaline
Received date: 2021-07-15
Revised date: 2021-08-20
Online published: 2021-09-03
Supported by
National Natural Science Foundation of China(21563025); National Natural Science Foundation of China(22162022)
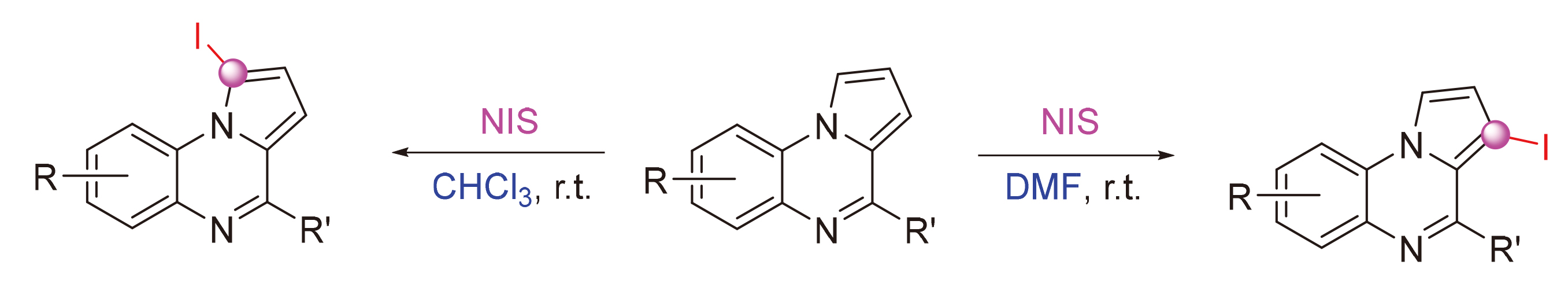
A solvent mediated regioselective C—H iodination of pyrrolo[1,2-a]quinoxaline with N-iodo-succininide (NIS) has been developed. 1-Iodopyrrolo[1,2-a]quinoxalines and 3-iodopyrrolo[1,2-a]quinoxalines could be selectively synthesized by using CHCl3 and N,N-dimethylformamide (DMF) as solvents, respectively. Furthermore, 1,3-dibromopyrroloquinoxalines were obtained as the main products by the bromination of pyrrolo[1,2-a]quinoxaline with N-bromo-succinimide (NBS). The method features simple and mild reaction conditions, good regioselectivity, broad substrate scope, and gram-scale synthesis. In addition, the further transformation of halogenated pyrrolo[1,2-a]quinoxaline products has also been investigated through palladium and iodine catalyzed C—X (X=C, S) bond formation reactions.
Yali Liu , Zhen Yang , Yang Li , Yan Liu , Ping Liu . Solvent Mediated Selective C—H Bond Iodination of Pyrrolo[1,2-a]quinoxaline[J]. Chinese Journal of Organic Chemistry, 2021 , 41(12) : 4789 -4797 . DOI: 10.6023/cjoc202107033
| [1] | (a) Ronga, L.; Del Favero, M.; Cohen, A.; Soum, C.; Le, Pape P.; Savrimoutou, S.; Pinaud, N.; Mullie, C.; Daulouede, S.; Vincendeau, P.; Farvacques, N.; Agnamey, P.; Pagniez, F.; Hutter, S.; Azas, N.; Sonnet, P.; Guillon, J. Eur. J. Med. Chem. 2014, 81, 378. |
| [1] | (b) van Heerden, L.; Cloete, T. T.; Breytenbach, J. W.; de Kock, C.; Smith, P.; Breytenbach, J. C.; N’Da, D. D. Eur. J. Med. Chem. 2012, 55, 335. |
| [1] | (c) Guillon, J.; Mouray, E.; Moreau, S.; Mullie, C.; Forfar, I.; Desplat, V.; Belisle-Fabre, S.; Pinaud, N.; Ravanello, F.; Le-Naour, A.; Le-Naour, Leger, J.-M.; Gosmann, G.; Jarry, C.; Deleris, G.; Sonnet, P.; Grellier, P. Eur. J. Med. Chem. 2011, 46, 2310. |
| [1] | (d) Jonet, A.; Guillon, J.; Mullie, C.; Cohen, A.; Bentzinger, G.; Schneider, J.; Taudon, N.; Hutter, S.; Azas, N.; Moreau, S.; Savrimoutou, S.; Agnamey, P.; Dassonville-Klimpt, A.; Sonnet, P. Med. Chem. 2018, 14, 293. |
| [1] | (e) Guillon, J.; Cohen, A.; Gueddouda, N. M.; Das, R. N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Monnier, A.; Monget, M.; Rubio, S.; Garnerin, T.; Azas, N.; Mergny, J.-L.; Mullie, C.; Sonnet, P. J. Enzyme Inhib. Med. Chem. 2017, 32, 547. |
| [2] | Xu, H.; Fan, L. Eur. J. Med. Chem., 2011, 46, 1919. |
| [3] | Morelli, E.; Gemma, S.; Budriesi, R.; Campiani, G.; Novellino, E.; Fattorusso, C.; Catalanotti, B.; Coccone, S. S.; Ros, S.; Borrelli, G.; Kumar, V.; Persico, M.; Fiorini, I.; Nacci, V.; Ioan, P.; Chiarini, A.; Hamon, M.; Cagnotto, A.; Mennini, T.; Fracasso, C.; Colovic, M.; Caccia, S.; Butini, S. J. Med. Chem. 2009, 52, 3548. |
| [4] | (a) Guillon, J.; Le Borgne, M.; Rimbault, C.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Baratin, S.; Marchivie, M.; Roche, S.; Bollacke, A.; Pecci, A.; Alvarez, L.; Despla, V.; Jose, J. Eur. J. Med. Chem. 2013, 65, 205. |
| [4] | (b) Desplat, V.; Moreau, S.; Gay, A.; Fabre, S. B.; Thiolat, D.; Massip, S.; Macky, G.; Godde, F.; Mossalayi, D.; Jarry, C.; Guillon, J. J. Enzyme Inhib. Med. Chem. 2010, 25, 204. |
| [5] | (a) Brindisi, M.; Brogi, S.; Maramai, S.; Grillo, A.; Borrelli, G.; Butini, S.; Novellino, E.; Allara, M.; Ligresti, A.; Campiani, G.; Di Marzo, V.; Gemma, S. RSC Adv. 2016, 6, 64651. |
| [5] | (b) Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Lesbordes, J.; Peyrilles, E.; Marchivie, M.; Routier, S.; Sonnet, P.; Rossi, F.; Ronga, L.; Guillon, J. Eur. J. Med. Chem. 2016, 113, 214. |
| [5] | (c) Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Rubio, S.; Pinaud, N.; Bigat, D.; Enriquez, E.; Marchivie, M.; Routier, S.; Sonnet, P.; Rossi, F.; Ronga, L.; Guillon, J. Chem- MedChem 2017, 12, 940. |
| [5] | (d) Wang, T.; Tang, Y.; Yang, Y.; An, Q.; Sang, Z.; Yang, T.; Liu, P.; Zhang, T.; Deng, Y.; Luo, Y.; Bioorg. Med. Chem. Lett. 2018, 28, 2084. |
| [5] | (e) Gemma, S.; Colombo, L.; Forloni, G.; Savini, L.; Fracasso, C.; Caccia, S.; Salmona, M.; Brindisi, M.; Joshi, B. P.; Tripaldi, P.; Giorgi, O.; Taglialatela-Scafati, E.; Novellino, I.; Fiorini, G.; Campiani, S.; Butini, S. Org. Biomol. Chem. 2011, 9, 5137. |
| [6] | (a) Huang, A.; Ma, C. Mini-Rev. Med. Chem. 2013, 13, 607. |
| [6] | (b) Campiani, G.; Aiello, F.; Fabbrini, M.; Morelli, E.; Ramunno, A.; Armoroli, S.; Nacci, V.; Garofalo, A.; Greco, G.; Novellino, E.; Maga, G.; Spadari, S.; Bergamini, A.; Ventura, L.; Bongiovanni, B.; Capozzi, M.; Bolacchi, F.; Marini, S.; Coletta, M.; Guiso, G.; Caccia, S. J. Med. Chem. 2001, 44, 305. |
| [6] | (c) Glennon, R. A.; Daoud, M. K.; Dukat, M.; Teitler, M.; Herrick-Davis, K.; Purohit, A.; Syed, H. Bioorg. Med. Chem. 2003, 11, 4449. |
| [6] | (d) Carta, A.; Loriga, M.; Paglietti, G.; Mattana, A.; Fiori, P. L.; Mollicotti, P.; Sechi, L.; Zanetti, S. Eur. J. Med. Chem. 2004, 39, 195. |
| [6] | (e) Guillon, J.; Forfar, I.; Mamani-Matsuda, M.; Desplat, V.; Saliège, M.; Thiolat, D.; Massip, S.; Tabourier, A.; Léger, J.-M.; Dufaure, B.; Haumont, G.; Jarry, C.; Mossalayi, D. Bioorg. Med. Chem. 2007, 15, 194. |
| [6] | (f) Moarbess, G.; Deleuze-Masquefa, C.; Bonnard, V.; Gayraud-Paniagua, S.; Vidal, J. R.; Bressolle, F.; Pinguet, P.; Bonnet, P. A. Bioorg. Med. Chem. 2008, 16, 6601. |
| [7] | (a) Kalinin, A. A.; Islamova, L. N.; Fazleeva, G. M. Chem. Heterocycl. Compd. 2019, 55, 584. |
| [7] | (b) Cong, W.; Wang, L.; Yu, F.; Li, J. Chin. J. Org. Chem. 2018, 38, 2866. |
| [8] | (a) Qin, Y.; Zhu, L.; Luo, S. Chem. Rev. 2017, 117, 9433. |
| [8] | (b) Ping, L.; Chung, D. S.; Bouffard, J.; Lee, S. G. Chem. Soc. Rev. 2017, 46, 4299. |
| [8] | (c) Liu, C. X.; Gu, Q.; You, S. L. Trends Chem. 2020, 2, 737. |
| [8] | (d) Hartwig, J. F.; Larsen, M. A. ACS Cent. Sci. 2017, 46, 4299. |
| [8] | (e) Zheng, Q.; Jiao, N. Chem. Soc. Rev. 2016, 45, 4590. |
| [8] | (f) Sun, K.; Xiao, F.; Yu, B.; He, W. Chin. J. Catal. 2021, 13, 63850. |
| [8] | (g) Xie, L.; Peng, S.; Yang, L.; Peng, C.; Lin, Y.; Yu, X.; Cao, Z.; Peng, Y.; He, W. Green Chem. 2021, 23, 374. |
| [8] | (h) Chen, J.; Wu, H.; Gui, Q.; Yan, S.; Deng, J.; Lin, Y.; Cao, Z.; He, W. Chin. J. Catal. 2021, 42, 1445. |
| [8] | (i) Chen, J.; Zhong, C.; Gui, Q.; Zhou, Y.; Fang, Y.; Liu, K.; Lin, Y.; Cao, Z.; He, W. Chin. Chem. Lett. 2021, 32, 475. |
| [8] | (j) Zhu, X.; Li, X.; Li, X. Org. Chem. Front. 2021, 8, 3128. |
| [8] | (k) Chen, Q; Yang, Y; Wang, X. Chin. J. Org. Chem. 2020, 40, 454. (in Chinese) |
| [8] | ( 陈倩雯, 杨耀成, 王霞, 有机化学, 2020, 40, 454.) |
| [8] | (l) Hao, W.; Wang, Y.; Yang, G.; Liu, Y. Chin. J. Org. Chem. 2017, 37, 2678. (in Chinese) |
| [8] | ( 郝文燕, 王昱赟, 杨国敏, 刘云云, 有机化学, 2017, 37, 2678.) |
| [8] | (m) Gan, Z.; Li, G.; Yang, X. Sci. China. Chem. 2020, 63, 1652. |
| [8] | (n) Sun, K.; Lv, Y.; Wang, J. Org. Lett. 2015, 17, 4408. |
| [8] | (o) Luo, J.; Xu, X.; Zhao, Y. Chin. J. Org. Chem. 2017, 37, 2873. (in Chinese) |
| [8] | ( 骆钧飞, 徐星, 赵延超, 梁洪泽, 有机化学, 2017, 37, 2873.) |
| [9] | Yang, Z.; He, J.; Wei, Y.; Li, W.; Liu, P.; Org. Biomol. Chem. 2020, 18, 3360. |
| [10] | Yang, Z.; He, J.; Wei, Y.; Li, W.; Liu, P.; Zhao, J.; Wei, Y. Org. Biomol. Chem. 2020, 18, 9088. |
| [11] | Le, H.; Hoang, T.; Tran, T.; Nguyen, C.; Chiem, L.; Phan, N.; Nguyen, T. Tetrahedron Lett. 2021, 67, 152879. |
| [12] | Liu, Y.; Wei, Y.; Yang, Z.; Li, Y.; Liu, Y.; Liu, P. Org. Biomol. Chem. 2021, 19, 5191. |
| [13] | Cheeseman, G. W. H.; Roy, P. D. J. Chem. Soc. C, 1968, 2848. |
| [14] | Reeves, J. T.; Fandrick, D. R.; Tan, Z.; Song, J. J.; Lee, H.; Yee, N. K.; Senanayake, C. H. J. Org. Chem. 2010, 75, 992. |
/
| 〈 |
|
〉 |