

Chinese Journal of Organic Chemistry >
Synthesis and Characterization of New Indeno[1,2-b]fluorene-6,12-dione Derivatives
Received date: 2022-06-21
Revised date: 2022-07-26
Online published: 2022-09-02
Supported by
National Natural Science Foundation of China(21302120)
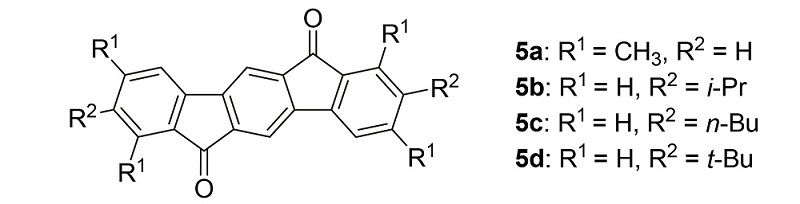
Indeno fluorene-6,12-dione derivatives 5 modified by different alkyl substitutions were designed and synthesized from 2,5-dibromo-p-xylene and phenylboronic acid derivatives through oxidation, esterification, Suzuki coupling, saponi- fication and cyclization reactions. Alkyl chains of different lengths were introduced into molecules to improve the solubility of the compounds and investigate the effects of systematic substitution variation on their properties. It was found that the solubility, thermal and optical stability of the new compounds were better than those of pentacene. Compounds 5 have increased electronegativities because of the low lowest unoccupied molecular orbital (LUMO) energies (≤–3.4 eV). 2,8- Diisopropylindeno[1,2-b]fluorene-6,12-dione (5b) and 2,8-dibutylindeno[1,2-b]fluorene-6,12-dione (5c) have rigid molecular frameworks and pack into slipped face-to-face π-stacks, the layer spacings are 0.3457 and 0.3423 nm, respectively. The molecular packing could facilitate carrier transport. The properties of these new materials indicate that they are good candidates for applications in organic optoelectronics.
Jing Wang , Linlin Wu , Qian Wang . Synthesis and Characterization of New Indeno[1,2-b]fluorene-6,12-dione Derivatives[J]. Chinese Journal of Organic Chemistry, 2023 , 43(1) : 223 -228 . DOI: 10.6023/cjoc202206038
| [1] | Jiang, H.; Zhu, S. L.; Cui, Z. D.; Li, Z. Y.; Liang, Y. Q.; Zhu, J. M.; Hu, Peng.; Zhang, H. L.; Hu, W. P. Chem. Soc. Rev. 2022, 51, 3071. |
| [2] | Jiang, H.; Hu, W. P. Angew. Chem., Int. Ed. 2020, 59, 1408. |
| [3] | Dong, H. L.; Zhu, H. F.; Meng, Q.; Gong, X.; Hu, W. P. Chem. Soc. Rev. 2012, 41, 1754. |
| [4] | Baeg, K. J.; Caironi, M.; Noh, Y. Y. Adv. Mater. 2013, 25, 4210. |
| [5] | Koo, J. H.; Kim, D. C.; Shim, H. J.; Kim, T. H.; Kim, D. H. Adv. Funct. Mater. 2018, 28, 1801834. |
| [6] | Zschieschang, U.; Yamamoto, T.; Takimiya, K.; Kuwabara, H.; Ikeda, M.; Sekitani, T.; Someya, T.; Klauk, H. Adv. Mater. 2011, 23, 654. |
| [7] | Zhan, Y. Q.; Mei, Y. F.; Zheng, L. R. J. Mater. Chem. C 2014, 2, 1220. |
| [8] | Zhang, C. C.; Chen, P. L.; Hu, W. P. Chem. Soc. Rev. 2015, 44, 2087. |
| [9] | Chen, D.; Pei, Q. B. Chem. Rev. 2017, 117, 11239. |
| [10] | Han, S. T.; Zhou, Y.; Roy, V. A. L. Adv. Mater. 2013, 25, 5425. |
| [11] | McNellis, E. R.; Schott, S.; Sirringhaus, H.; Sinova, J. Phys. Rev. Mater. 2018, 2, 074405. |
| [12] | Kuehne, A. J. C.; Gather, M. C. Chem. Rev. 2016, 116, 12823. |
| [13] | Liu, Y.; Yu, G.; Liu, Y. Q. Sci. China: Chem. 2010, 53, 779. |
| [14] | Cai, Z. X.; Awais, M. A.; Zhang, N.; Yu, L. P. Chem, 2018, 4, 2538. |
| [15] | Cinar, M. E.; Ozturk, T. Chem. Rev. 2015, 115, 3036. |
| [16] | Dai, X. X.; Cheng, X. D.; Kan, Z. P.; Xiao, Z. Y.; Duan, T. N.; Hu, C.; Lu, S. R. Chin. J. Org. Chem. 2020, 40, 4031. (in Chinese) |
| [16] | (戴学新, 成晓东, 阚志鹏, 肖泽云, 段泰男, 胡超, 陆仕荣, 有机化学, 2020, 40, 4031.) |
| [17] | Li, H. Y.; Tee, B. C. K.; Cha, J. J.; Cui, Y.; Chung, J. W.; Lee, S. Y.; Bao, Z. N. J. Am. Chem. Soc. 2012, 134, 2760. |
| [18] | Dong, H. L.; Hu, W. P. Acc. Chem. Res. 2016, 49, 2435. |
| [19] | Kitamura, M.; Arakawa, Y. J. Phys.: Condens. Matter 2008, 20, 184011. |
| [20] | Jurchescu, O. D.; Popinciuc, M.; van Wees, B. J.; Palstra, T. T. M. Adv. Mater. 2007, 19, 688. |
| [21] | Maliakal, A.; Raghavachari, K.; Katz, H.; Chandross, E.; Siegrist, T. Chem. Mater. 2004, 16, 4980. |
| [22] | Ebata, H.; Izawa, T.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H.; Yui, T. J. Am. Chem. Soc. 2007, 129, 15732. |
| [23] | Usta, H.; Risko, C.; Wang, Z.; Huang, H.; Deliomeroglu, M. K.; Zhukhovitskiy, A.; Facchetti, A.; Marks, T. J. J. Am. Chem. Soc. 2009, 131, 5586. |
| [24] | Fan, Z. P.; Li, X. Y.; Luo, X. E.; Fei, X.; Sun, B.; Chen, L. C.; Shi, Z. F.; Sun, C. L.; Shao, X. F.; Zhang, H. L. Adv. Funct. Mater. 2017, 27, 1702318. |
| [25] | Sung, H.; Lin, H. Macromolecules 2004, 37, 7945. |
| [26] | Hou, J. H.; Tan, Z. A.; Yan, Y.; He, Y. J.; Yang, C. H.; Li, Y. F. J. Am. Chem. Soc. 2006, 128, 4911. |
| [27] | Zhao, C. C.; Zhang, Y.; Ng, M. K. J. Org. Chem. 2007, 72, 6364. |
| [28] | Rose, B. D.; Santa Maria, P. J., Fix, A. G.; Vonnegut, C. L.; Zakharov, L. N.; Parkin, S. R. Michael M. H. Beilstein J. Org. Chem. 2014, 10, 2122. |
| [29] | Wang, J.; Zeng, W. L.; Xu, H.; Li, B.; Cao, X. P.; Zhang, H. L. Chin. J. Chem. 2012, 30, 681. |
| [30] | Gao, X. K.; Wu, W. P.; Liu, Y. Q.; Jiao, S. B.; Qiu, W. F.; Yu, G.; Wang, L. P.; Zhu, D. B. J. Mater. Chem. 2007, 17, 736 |
| [31] | Romain, M.; Chevrier, M.; Bebiche, S.; Mohammed-Brahim, T.; Rault-Berthelot, J.; Jacques, E.; Poriel, C. J. Mater. Chem. C 2015, 3, 5742. |
| [32] | Kobayashi, N.; Sasaki, M.; Nomoto, K. Chem. Mater. 2009, 21, 552. |
/
| 〈 |
|
〉 |