

Chinese Journal of Organic Chemistry >
Research and Application of N-Ts Cyanamides in Organic Synthesis
Received date: 2022-07-13
Revised date: 2022-08-08
Online published: 2022-09-15
Supported by
Natural Science Foundation of Henan Province(212300410152); Henan University of Animal Husbandry and Economy(2019HNUAHEDF011); Henan University of Animal Husbandry and Economy(XKYCXJJ2020006)
N-Ts cyanamide, which has been widely used in the construction of nitrogen-containing framework, is one of the efficient and practical multifunctional synthetic precursors in organic synthesis. The progress in application of N-Ts cyanamide according to the reaction types is summarized, including the application in cyanation, cyclization reaction via cyanamide anion, cyanamidation, sulfonylation and cyclization reaction via cyano-group. The future development direction of this field is also prospected.
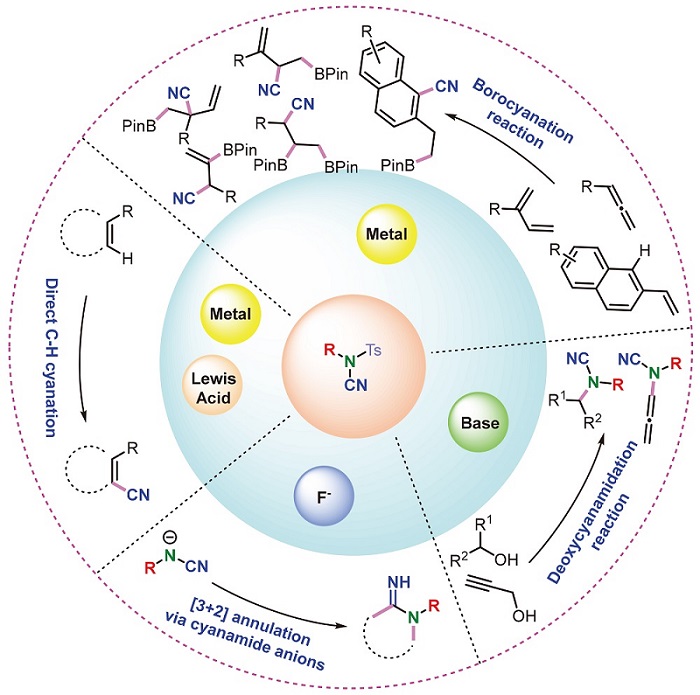
Key words: N-Ts cyanamides; cyanation; cyanamidation; sulfonylation; cyclization reactions
Chuanchuan Wang , Zhiwei Ma , Xuehui Hou , Longhua Yang , Yajing Chen . Research and Application of N-Ts Cyanamides in Organic Synthesis[J]. Chinese Journal of Organic Chemistry, 2023 , 43(1) : 74 -93 . DOI: 10.6023/cjoc202207022
| [1] | Frederick, K. J. J. Chem. Soc. 1949, 1034. |
| [2] | Anbarasan, P.; Neumann, H.; Beller, M. Angew. Chem. Int. Ed. 2011, 50, 519. |
| [3] | Cui, J.; Song, J.; Liu, Q.; Liu, H.; Dong, Y. Chem. Asian J. 2018, 13, 482. |
| [4] | Wang, R.; Falck, J. R. Chem. Commun. 2013, 49, 6516. |
| [5] | Gong, T.-J.; Xiao, B.; Cheng, W.-M.; Su, W.; Xu, J.; Liu, Z.-J.; Liu, L.; Fu, Y. J. Am. Chem. Soc. 2013, 135, 10630. |
| [6] | Chaitanya, M.; Yadagiri, D.; Anbarasan, P. Org. Lett. 2013, 15, 4960. |
| [7] | Gu, L.-J.; Jin, C.; Wang, R.; Ding, H.-Y. ChemCatChem 2014, 6, 1225. |
| [8] | Han, J.; Pan, C.; Jia, X.; Zhu, C. Org. Biomol. Chem. 2014, 12, 8603. |
| [9] | Dong, J.; Wu, Z.; Liu, Z.; Liu, P.; Sun, P. J. Org. Chem. 2015, 80, 12588. |
| [10] | Zhu, X.; Shen, X.-J.; Tian, Z.-Y.; Lu, S.; Tian, L.-L.; Liu, W.-B.; Song, B.; Hao, X.-Q. J. Org. Chem. 2017, 82, 6022. |
| [11] | Zhang, H.; Jing, Li, Zheng, Y.; Sang, R.; Zhao, Y.; Wang, Q.; Wu, Y. Eur. J. Org. Chem. 2018, 723. |
| [12] | Li, J.; Shi, L.; Zhang, S.-P.; Wang, X.-Y.; Zhu, X.; Hao, X.-Q.; Song, M.-P. J. Org. Chem. 2020, 85, 10835. |
| [13] | Lv, S.; Li, Y.; Yao, T.; Yu, X.; Zhang, C.; Hai, L.; Wu, Y. Org. Lett. 2018, 20, 4994. |
| [14] | Jia, J.; Liu, X.; Shi, J.; Xu, H. E.; Yi, W. Asian J. Org. Chem. 2015, 4, 1250. |
| [15] | Chaitanya, M.; Anbarasan, P. J. Org. Chem. 2015, 80, 3695. |
| [16] | Deng, C.; Sun, Y.; Ren, Y.; Zhang, W. Dalton Trans. 2019, 48, 168. |
| [17] | Mishra, N. K.; Jeong, T.; Sharma, S.; Shin, Y.; Han, S.; Park, J.; Oh, J. S.; Kwak, J. H.; Jung, Y. H.; Kim, I. S. Adv. Synth. Catal. 2015, 357, 1293. |
| [18] | Chaitanya, M.; Anbarasan, P. Org. Lett. 2015, 17, 3766-3769. |
| [19] | Su, W.; Gong, T.-J.; Xiao, B.; Fu, Y. Chem. Commun. 2015, 51, 11848. |
| [20] | Lu, X.; Huang, Y. Org. Chem. Front. 2021, 8, 3008. |
| [21] | Li, J.; Xu, W.; Ding, J.; Lee, K.-H. Tetrahedron Lett. 2016, 57, 1205. |
| [22] | Song, F.; Salter, R.; Chen, L. J. Org. Chem. 2017, 82, 3530. |
| [23] | Heydari, S.; Habibi, D.; Faraji, A. R.; Keypour, H.; Mahmoudabadi, M. Inorg. Chim. Acta 2021, 514, 119956. |
| [24] | Yang, Y.; Buchwald, S. L. Angew. Chem. Int. Ed. 2014, 53, 8677. |
| [25] | Yang, Y.; Liu, P. ACS Catal. 2015, 5, 2944. |
| [26] | Yang, Y. Angew. Chem. Int. Ed. 2016, 55, 345. |
| [27] | Zhao, W.; Montgomery, J. Angew. Chem. Int. Ed. 2015, 54, 12683. |
| [28] | Zhao, W.; Montgomery, J. J. Am. Chem. Soc. 2016, 138, 9763. |
| [29] | Jia, T.; He, Q.; Ruscoe, R. E.; Pulis, A. P.; Procter, D. J. Angew. Chem. Int. Ed. 2018, 57, 11305. |
| [30] | Wen, L.; Zhang, H.; Wang, J.; Meng, F. Chem. Commun. 2018, 54, 12832. |
| [31] | Jia, T.; Smith, M. J.; Pulis, A. P.; Perry, G. J. P.; Procter, D. J. ACS Catal. 2019, 9, 6744. |
| [32] | Li, Z.; Zhang, L.; Nishiura, M.; Luo, G.; Luo, Y.; Hou, Z. ACS Catal. 2020, 10, 11685. |
| [33] | Li, J.; Ackermann, L. Angew. Chem. Int. Ed. 2015, 54, 3635. |
| [34] | Yu, D.-G.; Gensch, T.; Azambuja, F.; Va?squez-Céspedes, S.; Glorius, F. J. Am. Chem. Soc. 2014, 136, 17722. |
| [35] | Cai, Y.; Qian, X.; Rérat, A.; Auffrant, A.; Gosmini, C. Adv. Synth. Catal. 2015, 357, 3419. |
| [36] | Liu, W.; Ackermann, L. Chem. Commun. 2014, 50, 1878. |
| [37] | Mishra, A.; Vats, T. K.; Deb, I. J. Org. Chem. 2016, 81, 6525. |
| [38] | Li, H.; Chen, J.; Dong, J.; Kong, W. Org. Lett. 2021, 23, 6466. |
| [39] | Liu, W.; Richter, S. C.; Mei, R.; Feldt, M.; Ackermann, L. Chem. Eur. J. 2016, 22, 17958. |
| [40] | Yu, X.; Tang, J.; Jin, X.; Yamamoto, Y.; Bao, M. Asian J. Org. Chem. 2018, 7, 550. |
| [41] | Anbarasan, P.; Neumann, H.; Beller, M. Chem. Eur. J. 2011, 17, 4217. |
| [42] | Yang, Y.; Zhang, Y.; Wang, J. Org. Lett. 2011, 13, 5608. |
| [43] | Kiyokawa, K. Nagata, T.; Minakata, S. Angew. Chem. Int. Ed. 2016, 55, 10458. |
| [44] | Benn, K.; Nicholson, K.; Langer, T.; Thomas, S. P. Chem. Commun. 2021, 57, 9406. |
| [45] | Kiyokawa, K.; Hata, S.; Kainuma, S.; Minakata, S. Chem. Commun. 2019, 55, 458. |
| [46] | Zhang, W.; Li, T.; Wang, Q.; Zhao, W. Adv. Synth. Catal. 2019, 361, 4914. |
| [47] | Ren, X.; Shen, C.; Wang, G.; Shi, Z.; Tian, X.; Dong, K. Org. Lett. 2021, 23, 2527. |
| [48] | Bhat. S. V.; Robinson, D.; Moses, J. E.; Sharma, P. Org. Lett. 2016, 18, 1100. |
| [49] | Sharma, P.; Bhat, S. V.; Prabhath, M. R. R.; Molino, A.; Nauha, E.; Wilson, D. J. D.; Moses, J. E. Org. Lett. 2018, 20, 4263. |
| [50] | Wang, C.-C.; Qu, Y.-L.; Liu, X.-H.; Ma, Z.-W.; Yang, B.; Liu, Z.-J.; Chen, X.-P.; Chen, Y.-J. J. Org. Chem. 2021, 86, 3546. |
| [51] | Wang, C.-C.; Wang, X.-L.; Zhang, Q.-L.; Liu, J.; Ma, Z.-W.; Liu, Z.-J.; Chen, Y.-J. Org. Chem. Front. 2022, 9, 1574. |
| [52] | Ayres, J. N.; Ashford, M. W.; St?ckl, Y.; Prudhomme, V.; Ling, K. B.; Platts, J. A.; Morrill, L. C. Org. Lett. 2017, 19, 3835. |
| [53] | Ayres, J. N.; Williams, M. T. J.; Tizzard, G. J.; Coles, S. J.; Ling, K. B.; Morrill, L. C. Org. Lett. 2018, 20, 5282. |
| [54] | Li, J.-S.; Yang, P.-P.; Chen, G.-Q.; Xie, X.-Y.; Li, Z.-W.; Li, W.-S.; Liu, W.-D. Asian J. Org. Chem. 2019, 8, 246. |
| [55] | Kasthuri, M.; Babu, H. S.; Kumar, K. S.; Sudhakar, C.; Kumar, P. V. N. Synlett 2015, 26, 897. |
| [56] | ?lachtová, V.; Chasák, J.; Brulíková, L. ACS Omega 2019, 4, 19314. |
| [57] | Murthy, V. N.; Nikumbh, S. P.; Kumar, S. P.; Rao, L. V.; Raghunadh, A. Tetrahedron Lett. 2015, 56, 5767. |
/
| 〈 |
|
〉 |