

Chinese Journal of Organic Chemistry >
Synthesis of Quinoline Derivatives by Friedländer Reaction Catalyzed by Ruthenium Complexes of Substituted 8-Hydroxyquinoline
Received date: 2022-10-28
Revised date: 2022-12-02
Online published: 2023-02-15
Supported by
National Natural Science Foundation of China(22072099); Chunhui Program of Ministry of Education(192635)
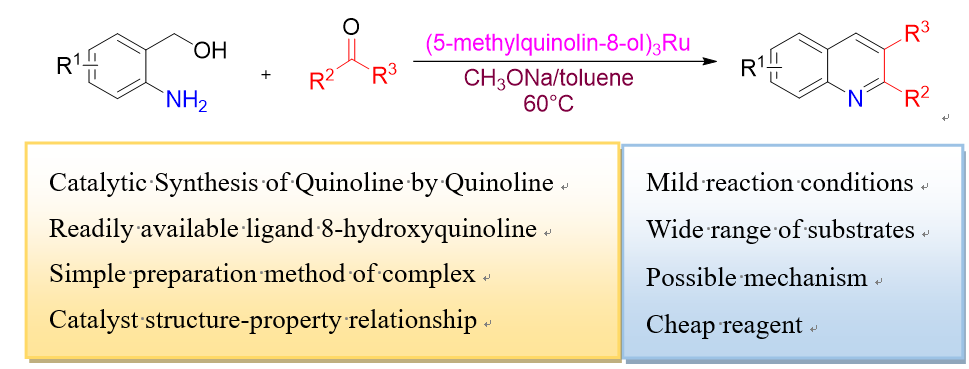
The Friedländer quinoline synthesis method is a reaction of o-aminoaryl aldehyde or ketone with methyl ketone to obtain quinoline. In this paper, a method for synthesizing quinoline catalyzed by ruthenium complexes of quinoline was reported. Using 8-hydroxyquinoline ruthenium complex as catalyst, the reaction conditions were optimized. The effects of different substituents of 8-hydroxyquinoline ruthenium complexes on the reaction yield were comparatively studied. Among them, 5-methyl-8-hydroxyquinoline (1e) ruthenium complex catalyzed the synthesis of 2-phenylquinoline from o-aminobenzyl alcohol and acetophenone with the highest yield of 73%. The relationship between ligand structure and catalytic performance was discussed by combining IR, UV and density functional theory (DFT) calculations. A possible mechanism was proposed, which included the formation of aldehyde transition state through β-H elimination, cross aldol reaction, imination cyclization and finally dehydration to produce the target product. Using (1e)3Ru as catalyst, 32 quinoline derivatives with different substitutions were synthesized with 69%~94% yields under the optimized reaction conditions, which confirmed the generality of this method.
Yue Zhu , Lu Chen , Jing Zhao , Qingrong Sun , Weiqing Yang , Haiyan Fu , Menglin Ma . Synthesis of Quinoline Derivatives by Friedländer Reaction Catalyzed by Ruthenium Complexes of Substituted 8-Hydroxyquinoline[J]. Chinese Journal of Organic Chemistry, 2023 , 43(7) : 2528 -2542 . DOI: 10.6023/cjoc202210036
| [1] | Zhu, Y.; Cai, C. RSC Adv. 2014, 4, 52911. |
| [2] | Pothikumar, R.; Bhat, V. T.; Namitharan, K. Chem. Commun. 2020, 56, 13607. |
| [3] | Akbari, J.; Heydari, A.; Kalhor, H. R.; Kohan, S. A. Cheminform 2010, 41, 137. |
| [4] | Das, S.; Sinha, S.; Samanta, D.a; Mondal, R.; Chakraborty, G.; Brandao, P.; Paul, N. D. J. Org. Chem. 2019, 84, 10160. |
| [5] | Mondal, R.; Chakraborty, G.; Guin, A. K.; Pal, S.; Paul, N. D. Tetrahedron 2021, 100, 132479. |
| [6] | (a) Genc, S.; Arslan, B.; Gulcemal, S.; Gunnaz, S.; Cetinkaya, B.; Gulcemal, D. J. Org. Chem. 2019, 84, 6286. |
| [6] | (b) Wang, R.; Fan, H.; Zhao, W.; Li, F. Org. Lett. 2016, 18, 3558. |
| [7] | Das, S.; Maiti, D.; Sarkar, D. S. J. Org. Chem. 2018, 83, 2309. |
| [8] | Zhang, G.; Wu, J.; Zeng, H.; Zhang, S.; Yin, Z.; Zheng, S. Org. Lett. 2017, 19, 1080. |
| [9] | Cho, C. S.; Seok, H. J.; Shim, S. C. J. Heterocycl. Chem. 2005, 42, 1219. |
| [10] | Mahajan, A.; Arya, A.; Chundawat, T. S. Synth. Commun. 2019, 49, 1926. |
| [11] | (a) Mierde, H. V.; Voort, P. V. D.; Vos, D. D.; Verpoort, F. Eur. J. Org. Chem. 2008, 1625. |
| [11] | (b) Mierde, H. V.; Ldoux, N.; Allaert, B.; Voort, P. V. D.; Drozdzak, R.; Vos, D. D.; Verpoort, F. New J. Chem. 2007, 31, 1572. |
| [12] | Yun, X. J.; Zhu, J. W.; Yan, J. Deng, W.; Yao, Z. J. Inorg. Chem. 2020, 59, 7841. |
| [13] | Huo, S.; Kong, S.; Zeng, G.; Feng, Q.; Hao, Z.; Han, Z.; Lin, J.; Lu, G. L. J. Mol. Catal. A: Chem. 2021, 514, 111773. |
| [14] | Verma, A.; Hazra, S.; Dolui, P.; Elias, A. J. J. Org. Chem. 2021, 10, 626. |
| [15] | (a) Zhang, Y.; Cheng, H.; Sun, Q.; Chen, H.; Yang, W.; Ma, M. J. Chem. Res. 2021, 45, 623. |
| [15] | (b) He, J.; Zhou, T.; Cao, Y.; Zhang, Y.; Yang, W.; Ma, M. J. Fluoresc. 2018, 28, 1121. |
| [15] | (c) Alam, M. N.; Moni, M. A.; Yu, J. Q; Beale, P.; Turner, P.; Proschogo, N.; Rahman, M. A.; Hossain, M. P.; Huq, P. Int. J. Mol. Sci. 2021, 22, 8471. |
| [16] | Rodman, G. S.; Nagle, J. K. Inorg. Chim. Acta 1985, 105, 205. |
| [17] | (a) Vander, M. H.; Voot, P. V. D.; Vos, D. D.; Verpoort, F. Eur. J. Org. Chem. 2008, 1625. |
| [17] | (b) Subramanian, M.; Sundar, S.; Rengan, R. Appl. Organomet. Chem. 2018, 32, e4582. |
| [18] | Ghosh, T. N.; Lasker, S. L.; Banerjee, S. J. Indian Chem. Soc. 1944, 21, 354. |
| [19] | Bronson, R. T.; Montalti, M.; Prodi, L.; Zaccheroni, N.; Lamb, R. D.; Dalley, N. K.; Izatt, R. M.; Bradshaw, J. S.; Savage, P. B. Tetrahedron 2004, 60, 11139. |
| [20] | Khusnutdinov, R. I.; Bayguzina, A. R.; Aminov, R. I. Russ. J Gen. Chem. 2016, 86, 1613. |
| [21] | Thinnes, C. C.; Tumber, A.; Yapp, C.; Scozzafava, G.; Yeh, T.; Chan, M. C.; Tran, T. A.; Hsu, K.; Tarhonskaya, H.; Walport, L. J.; Wilkins, S. E.; Martinez, E. D.; Muller, S.; Pugh, C. W.; Ratcliffe, P. J.; Brennan, P. E.; Kawamura, A.; Schofield, C. J. Chem. Commun. 2015, 51, 15458. |
| [22] | Phillips, J. P.; Elbinger, R. L.; Merritt, L. L. J. Am. Chem. Soc. 1949, 71, 3986. |
| [23] | Warner, V. D.; Sane, J. N.; Mirth, D. B.; Turesky, S. S.; Soloway, B. J. Med. Chem. 1976, 19, 167. |
| [24] | Mirkovic, B.; Renko, M.; Turk, S.; Sosic, I.; Jevnikar, Z.; Obermajer, N.; Turk, D.; Gobec, S.; Kos, J. ChemMedChem 2011, 6, 1351. |
| [25] | Lauer, W. M.; Arnold, R. T.; Tiffany, B.; Tinker, J. J. Am. Chem. Soc. 1946, 68, 1268. |
| [26] | Parua, S.; Sikari, R.; Sinha, S.; Das, S.; Chakraborty, G.; Paul, N. D. Org. Biomol. Chem. 2018, 16, 274. |
| [27] | Xi, L; Zhang, R.; Zhang, L.; Chen, S.; Yu, X. Org. Biomol. Chem. 2015, 13, 3924. |
| [28] | Xu, T.; Shao, Y.; Dai, L.; Yu, S.; Cheng, T.; Chen, J. J. Org. Chem. 2019, 84, 13604. |
| [29] | Saxena, J. P.; Stafford, W. H.; Stafford, W. L. J. Chem. Soc. 1959, 1579. |
| [30] | Qu, F; He, P.; Hu, R.; Cheng, X.; Wang, S.; Wu, J. Synth. Commun. 2015, 45, 2802. |
| [31] | Xu, J.; Sun, J.; Zhao, J.; Huang, B.; Li, X.; Sun, Y. RSC Adv. 2017, 7, 36242. |
/
| 〈 |
|
〉 |