

Chinese Journal of Organic Chemistry >
Recent Progress in Transition Metal Catalyzed C(sp3)—H Nitrene Insertion Reactions Assisted by Directing Groups
Received date: 2022-11-30
Revised date: 2023-02-01
Online published: 2023-02-15
Supported by
National Natural Science Foundation of China(91956103)
The transition metal catalyzed activation of C(sp3)—H bonds to form carbon-nitrogen bonds has been a challenging and hot research area. The transition metal-catalyzed C(sp3)—H amination strategy provides a more direct and efficient way to form C—N bonds. With the vigorous development in the field of C—H bond activation, a variety of directing groups and transition metal catalyst systems have been developed, which provide effective tools for C(sp3)—H amination through the inner sphere mechanism. In recent years, important progress has been made in directing group-assisted C(sp3)—H bond amination reactions, and many catalyst systems based on different transition metals with cheap and efficient amination reagents have been developed. The research progress of transition metal-catalyzed C(sp3)—H nitrene insertion reaction assisted by directing groups in the past decade is reviewed.
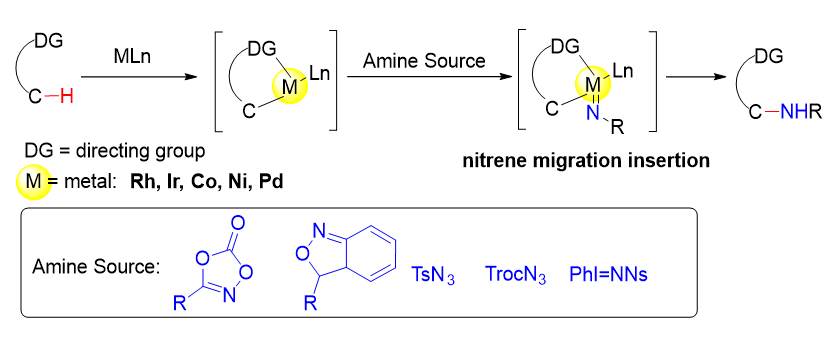
Key words: directing group; nitrene insertion; transition metal; C(sp3)—H; C—N bond formation
Xing Yang , Xu Liu , Lijia Wang . Recent Progress in Transition Metal Catalyzed C(sp3)—H Nitrene Insertion Reactions Assisted by Directing Groups[J]. Chinese Journal of Organic Chemistry, 2023 , 43(3) : 914 -923 . DOI: 10.6023/cjoc202211041
| [1] | Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences, Wiley-VCH, Weinheim, 2008. |
| [2] | (a) Zhao, J.; Zhang, Q. Acta Chim. Sinica 2015, 73, 1235. (in Chinese) |
| [2] | (赵金钵, 张前, 化学学报, 2015, 73, 1235.) |
| [2] | (b) Li, J.; Zhang, Y. Nat. Rev. Chem. 2022, 6, 303. |
| [2] | (c) Ma, J.-L.; Zhou, X.-M.; Guo, P.-H.; Cheng, H.-C.; Ji, H.-B. Chin. J. Chem. 2022, 40, 1204. |
| [2] | (d) Saranya, P. V.; Neetha, M.; Philip, R. M. Tetrahedron 2022, 104, 132582. |
| [2] | (e) Hwang, H.; Kim, J.; Jeong, J.; Chang, S. J. Am. Chem. Soc. 2014, 136, 10770. |
| [3] | (a) Davies, H. M. L.; Manning, J. R. Nature 2008, 451, 417. |
| [3] | (b) Davies, H. M. L.; Liao, K.-B. Nat. Rev. Chem. 2019, 3, 347. |
| [3] | (c) Du, B.; Chan, C.-M.; Au, C.-M.; Yu, W.-Y. Acc. Chem. Res. 2022, 55, 2123. |
| [3] | (d) Liang, H.; Wang, J. Chem.-Eur. J. 2022, 28, e202202461. |
| [4] | For review: (a) Godula, K.; Sames, D., Science 2006, 312, 67. |
| [4] | (b) Dick, A. R.; Sanford, M. S. Tetrahedron 2006, 62, 2439. |
| [4] | (c) Zalatan, D. N.; Du Bois, J. Top. Curr. Chem. 2010, 292, 347. |
| [4] | (d) Driver, T. G. Org. Biomol. Chem. 2010, 8, 3831. |
| [4] | For leading references: |
| [4] | (e) Zhang, S.-Y.; He, G.; Nack, W. A.; Zhao, Y.; Li, Q.; Chen, G. J. Am. Chem. Soc. 2013, 135, 2124. |
| [4] | (f) He, J.; Wasa, M.; Chan, K. S. L.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 3387. |
| [4] | (g) Zhang, S.-Y.; Li, Q.; He, G.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2013, 135, 12135. |
| [4] | (h) Shan, G.; Yang, X.; Zong, Y.; Rao, Y. Angew. Chem., Int. Ed. 2013, 52, 13606. |
| [4] | (i) Chen, K.; Hu, F.; Zhang, S.-Q.; Shi, B.-F. Chem. Sci. 2013, 4, 3906. |
| [4] | (j) Rodríguez, N.; Romero-Revilla, J. A.; Fernández-Ibá?ez, M. á.; Carretero, J. C. Chem. Sci. 2013, 4, 175. |
| [4] | (k) Aihara, Y.; Chatani, N. J. Am. Chem. Soc. 2014, 136, 898. |
| [4] | (l) Wu, X.; Zhao, Y.; Ge, H. J. Am. Chem. Soc. 2014, 136, 1789 |
| [5] | Wang, H.; Tang, G.; Li, X. Angew. Chem., Int. Ed. 2015, 54, 13049. |
| [6] | Dong, Y.; Chen, J.; Xu, H. Chem. Commun. 2018, 54, 11096. |
| [7] | Antien, K.; Geraci, A.; Parmentier, M.; Baudoin, O. Angew. Chem., Int. Ed. 2021, 60, 22948. |
| [8] | Barsu, N.; Rahman, M. A.; Sen, M.; Sundararaju, B. Chem.-Eur. J. 2016, 22, 9135. |
| [9] | Fukagawa, S.; Kojima, M.; Yoshino, T.; Matsunaga, S. Angew. Chem., Int. Ed. 2019, 58, 18154. |
| [10] | Kato, Y.; Lin, L.; Kojima, M.; Yoshino, T.; Matsunaga, S. ACS Catal. 2021, 11, 4271. |
| [11] | Tan, P. W.; Mak, A. M.; Sullivan, M. B.; Dixon, D. J.; Seayad, J. Angew. Chem., Int. Ed. 2017, 56, 16550. |
| [12] | Fukagawa, S.; Kato, Y.; Tanaka, R.; Kojima, M.; Yoshino, T.; Matsunaga, S. Angew. Chem., Int. Ed. 2019, 58, 1153. |
| [13] | Sekine, D.; Ikeda, K.; Fukagawa, S.; Kojima, M.; Yoshino, T.; Matsunaga, S. Organometallics 2019, 38, 3921. |
| [14] | Shi, H.; Dixon, D. J. Chem. Sci. 2019, 10, 3733. |
| [15] | Mahato, S. K.; Ohara, N.; Khake, S. M.; Chatani, N. I. ACS Catal. 2021, 11, 7126. |
| [16] | Du, B.; Ouyang, Y.; Chen, Q.; Yu, W.-Y. J. Am. Chem. Soc. 2021, 143, 14962 |
| [17] | Kang, T.; Kim, Y.; Lee, D.; Wang, Z.; Chang, S. J. Am. Chem. Soc. 2014, 136, 4141. |
| [18] | Zhang, T.; Hu, X.; Dong, X.; Li, G.; Lu, H. Org. Lett. 2018, 20, 6260. |
| [19] | Han, J.-L.; Qin, Y.; Zhao, D. ACS Catal. 2019, 9, 6020. |
| [20] | Kim, Y. B.; Won, J.; Lee, J.; Kim, J.; Zhou, B.; Park, J.-W.; Baik, M.-H.; Chang, S. ACS Catal. 2021, 11, 3067. |
| [21] | Xiao, X.; Hou, C.; Zhang, Z.; Ke, Z.; Lan, J.; Jiang, H.; Zeng, W. Angew. Chem., Int. Ed. 2016, 55, 11897. |
| [22] | Huang, X.; Wang, Y.; Lan, J.; You, J. Angew. Chem., nt. Ed. 2015, 54, 9404. |
| [23] | Liu, B.; Xie, P.; Zhao, J.; Wang, J.; Wang, M.; Jiang, Y.; Chang, J.; Li, X. Angew. Chem., Int. Ed. 2021, 60, 8396. |
| [24] | Wang, Y.; Liu, H.; Li, B.; Wang, B. Adv. Synth. Catal. 2019, 361, 1564. |
| [25] | Thu, H.-Y.; Yu, W.-Y.; Che, C.-M. J. Am. Chem. Soc. 2006, 128, 9048. |
| [26] | Yu, S.; Tang, G.; Li, Y.; Zhou, X.; Lan, Y.; Li, X. Angew. Chem., Int. Ed. 2016, 55, 8696. |
| [27] | Tang, C.; Zou, M.; Liu, J.; Wen, X.; Sun, X.; Zhang, Y.; Jiao, N. Chem.-Eur. J. 2016, 22, 11165. |
| [28] | Liu, R.-H.; Shan, Q.-C.; Hu, X.-H.; Loh, T.-P. Chem. Commun. 2019, 55, 5519. |
/
| 〈 |
|
〉 |