

Chinese Journal of Organic Chemistry >
Electrochemical Synthesis of Masked Organoboronic Acids RB(dan) at Room Temperature
Received date: 2022-11-27
Revised date: 2023-02-19
Online published: 2023-03-17
Supported by
National Natural Science Foundation of China(21662045); National Natural Science Foundation of China(11547233); Applied Basic Research Project of Yunnan Province(202101AT070079)
An electrochemical room-temperature synthesis of masked organoboronic acids RB(dan) (R=alkyl or aryl) has been developed. At room temperature, 1,8-diaminonaphthalene and boronic acids can smoothly participate in this reaction to provide a wide variety of RB(dan) (R=alkyl or aryl) in an undivided cell. The transformation has broad substrate scope and avoids using high-temperature or transition-metal catalysts, making it more sustainable and renewable.
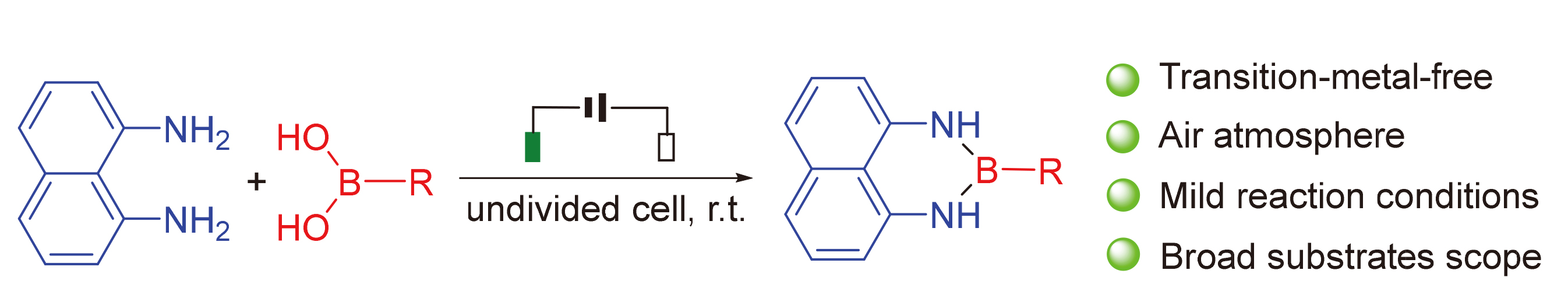
Key words: electrochemical synthesis; boronic acids; masked group; room temperature
Junying Zhang , Xiaojing Zhao , Ganpeng Li , Yonghui He . Electrochemical Synthesis of Masked Organoboronic Acids RB(dan) at Room Temperature[J]. Chinese Journal of Organic Chemistry, 2023 , 43(5) : 1815 -1823 . DOI: 10.6023/cjoc202211033
| [1] | Oh-e, T.; Miyaura, N.; Suzuki, A. Synlett 1990, 1990, 221. |
| [2] | Lennox, A. J. J.; Lloyd-Jones, G. C. Chem. Soc. Rev. 2014, 43, 412. |
| [3] | Noguchi, H.; Shioda, T.; Chou, C. M.; Suginome, M. Org. Lett. 2008, 10, 377. |
| [4] | Noguchi, H.; Hojo, K.; Suginome, M. J. Am. Chem. Soc. 2007, 129, 758. |
| [5] | Iwadate, N.; Suginome, M. J. Am. Chem. Soc. 2010, 132, 2548. |
| [6] | Iwadate, N.; Suginome, M. J. Organomet. Chem. 2009, 694, 1713. |
| [7] | Iwadate, N.; Suginome, M. Org. Lett. 2009, 11, 1899. |
| [8] | Wan, W. M.; Tian, D.; Jing, Y. N.; Zhang, X. Y.; Wu, W.; Ren, H.; Bao, H. L. Angew. Chem., Int. Ed. 2018, 57, 15510. |
| [9] | Li, J. Q.; Grillo, A. S.; Burke, M. D. Acc. Chem. Res. 2015, 48, 2297. |
| [10] | Xu, L.; Li, P. F. Chem. Commun. 2015, 51, 5656. |
| [11] | Yoshida, H.; Takemoto, Y.; Kamio, S.; Osaka, I.; Takaki, K. Org. Chem. Front. 2017, 4, 1215. |
| [12] | Yoshida, H.; Murashige, Y.; Osaka, I. Org. Synth. 2018, 95, 218. |
| [13] | Knapp, D. M.; Gillis, E. P.; Burke, M. D. J. Am. Chem. Soc. 2009, 131, 6961. |
| [14] | Dick, G. R.; Woerly, E. M.; Burke, M. D. Angew. Chem., Int. Ed. 2012, 51, 2667. |
| [15] | Chissick, S. S.; Dewar, M. J. S.; Maitlis, P. M. J. Am. Chem. Soc. 1961, 83, 2708. |
| [16] | Kaupp, G.; Naimi-Jamal, M. R.; Stepanenko, V. Chemistry 2003, 9, 4156. |
| [17] | Hackney, H. E.; Paladino, M.; Fu, H.; Hall, D. G. Chem.-Eur. J. 2020, 26, 14324. |
| [18] | Liao, S.; Hu, X.; Li, Y.; Wang, X.; Li, D.; Wang, Q.; Wang, Y.; Huang, X.; Xu, P.; Wu, H.; Li, X.; Yuan, J. Tetrahedron 2021, 90, 132205. |
| [19] | Slabber, C. A.; Grimmer, C. D.; Robinson, R. S. J. Organomet. Chem. 2013, 723, 122. |
| [20] | Pucheault, M.; Wood, J.; Marciasini, L.; Vaultier, M. Synlett 2014, 25, 551. |
| [21] | Yuan, Y.; Yang, J.; Lei, A. Chem. Soc. Rev. 2021, 50, 10058. |
| [22] | Wang, F.; Stahl, S. S. Acc. Chem. Res. 2020, 53, 561. |
| [23] | Jiao, K.-J.; Xing, Y.-K.; Yang, Q.-L.; Qiu, H.; Mei, T.-S. Acc. Chem. Res. 2020, 53, 300. |
| [24] | Xiong, P.; Xu, H.-C. Acc. Chem. Res. 2019, 52, 3339. |
| [25] | Horn, E. J.; Rosen, B. R.; Baran, P. S. ACS Cent. Sci. 2016, 2, 302. |
| [26] | Wu, Y.-C.; Jiang, S.-S.; Song, R.-J.; Li, J.-H. Chem. Commun. 2019, 55, 4371. |
| [27] | He, M.-X.; Mo, Z.-Y.; Wang, Z.-Q.; Cheng, S.-Y.; Xie, R.-R.; Tang, H.-T.; Pan, Y.-M. Org. Lett. 2020, 22, 724. |
| [28] | Zeng, L.; Li, J.; Gao, J.; Huang, X.; Wang, W.; Zheng, X.; Gu, L.; Li, G.; Zhang, S.; He, Y. Green Chem. 2020, 22, 3416. |
| [29] | He, Y.; Zeng, L.; Li, M.; Gu, L.; Zhang, S.; Li, G. J. Org. Chem. 2022, 87, 12622. |
| [30] | Wang, Y.; Zhao, X.-J.; Wu, X.; Zhang, L.; Li, G.; He, Y. ChemElectroChem 2022, 9, e202200378. |
| [31] | Wei, B.; Qin, J.-H.; Yang, Y.-Z.; Xie, Y.-X.; Ouyang, X.-H.; Song, R.-J. Org. Chem. Front. 2022, 9, 816. |
| [32] | Wu, Z.-L.; Chen, J.-Y.; Tian, X.-Z.; Ouyang, W.-T.; Zhang, Z.-T.; He, W.-M. Chin. Chem. Lett. 2022, 33, 1501. |
| [33] | Yang, D.; Yan, Q.; Zhu, E.; Lv, J.; He, W.-M. Chin. Chem. Lett. 2022, 33, 1798. |
| [34] | Guo, S.; Liu, L.; Hu, K.; Sun, Q.; Zha, Z.; Yang, Y.; Wang, Z. Chin. Chem. Lett. 2021, 32, 1033. |
| [35] | Hu, K.; Zhang, Y.; Zhou, Z.; Yang, Y.; Zha, Z.; Wang, Z. Org. Lett. 2020, 22, 5773. |
| [36] | Zeng, D.; Zhang, L.; Wang, W.; Li, G.; Zhao, X.-J.; He, Y. Eur. J. Org. Chem. 2022, e202200679. |
/
| 〈 |
|
〉 |