

Chinese Journal of Organic Chemistry >
Lanthanum Silylamide-Catalyzed Synthesis of Enol Phosphates
Received date: 2023-06-15
Revised date: 2023-07-13
Online published: 2023-07-27
Supported by
Natural Science Foundation of the Jiangsu Higher Education Institutions(18KJA150007)
Enol phosphate has important applicative value in many fields. The development of a catalytic and selective method for the synthesis of enol phosphate is of great significance. The reaction of 4-oxo-4-arylbut-2-enoate and diethyl phosphite catalyzed by lanthanum silylamide [(Me3Si)2N]3La(μ-Cl)Li(THF)3 is developed, which provides a series of enol phosphates in cis-configuration. Research on the mechanism indicates that the reaction undergoes a relatively rare [1,4]-phospha-Brook rearrangement. The reaction has advantages of ready availability of catalyst and high selectivity for alkenyl configuration, providing an efficient method for the synthesis of enol phosphates.
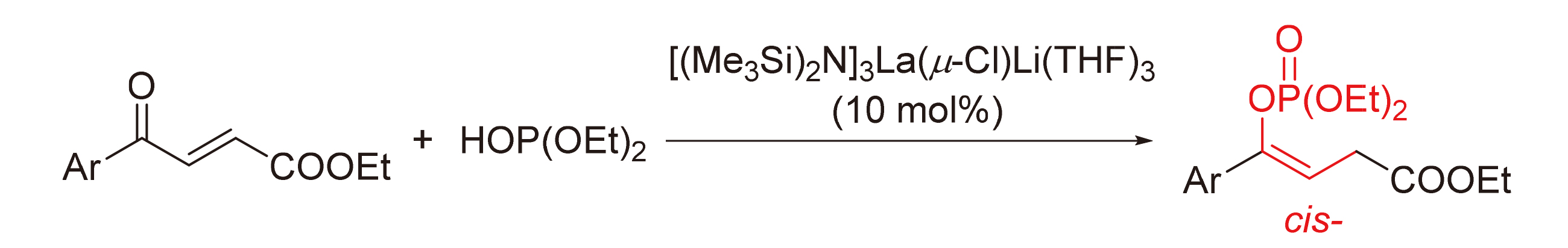
Kang Pan , Fan Xu . Lanthanum Silylamide-Catalyzed Synthesis of Enol Phosphates[J]. Chinese Journal of Organic Chemistry, 2023 , 43(12) : 4261 -4267 . DOI: 10.6023/cjoc202306012
| [1] | (a) Lichtenthaler F. W. Chem. Rev. 1961, 61, 607. |
| [1] | (b) Ternan N. G.; McGrath J. W.; Quinn J. P. Appl. Environ. Microbiol. 1998, 64, 2291. |
| [1] | (c) Zhang G.; Dai J.; Lu Z.; Dunaway-Mariano D. J. Biol. Chem. 2003, 278, 41302. |
| [1] | (d) Allison M.; Hutton R. D.; Cochrane F. C.; Yeoman J. A.; Jameson G. B.; Parker E. J. Biochemistry 2011, 50, 3686. |
| [2] | For selected reviews, see: (a) Li, W.; Wang, Z. RSC Adv. 2013, 3, 25565. |
| [2] | (b) Sellars J. D.; Steel P. G. Chem. Soc. Rev. 2011, 40, 5170. |
| [2] | (c) Li B.; Yu D.; Sun C.; Shi Z. Chem.-Eur. J. 2011, 17, 1728. |
| [2] | For selected recent papers see: |
| [2] | (d) Hu X.; Yang X.; Loh T. Angew. Chem., Int. Ed. 2015, 54, 15535. |
| [2] | (e) Fuwa H.; Sasaki M. J. Org. Chem. 2009, 74, 212. |
| [2] | (f) Sasaki M.; Fuwa H. Synlett 2004, 1851. |
| [2] | (g) Sasaki M.; Ishikawa M.; Fuwa H.; Tachibana K. Tetrahedron 2002, 58, 1889. |
| [2] | (h) Skowronska A.; Koprowski M.; Krawczyk E. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 1877. |
| [3] | (a) Senra J. D.; Silva A. C.; Santos R. V.; Malta L. F. B.; Simas A. B. C. J. Chem. 2017, 8418939. |
| [3] | (b) Kerr W. J.; Lindsay D. M.; Patel V. K.; Rajamanickam M. Org. Biomol. Chem. 2015, 13, 10131. |
| [4] | Sosa J. R.; Tudjarian A. A.; Minehan T. G. Org. Lett. 2008, 10, 5091. |
| [5] | (a) Guo H.; Zhang Y.; Li Z.; Zhao P.; Li N.; Shi E. RSC Adv. 2022, 12, 14844. |
| [5] | (b) Cao Y.; Gao Z.; Li J.; Bi X.; Yuan L.; Pei C.; Guo Y.; Shi E. RSC Adv. 2020, 10, 29493. |
| [6] | Ghomri A.; Atmani A. C. R. Chimie 2014, 17, 1230. |
| [7] | Lee P. H.; Kim S.; Park A.; Chary B. C.; Kim S. Angew. Chem., Int. Ed. 2010, 49, 6806. |
| [8] | Zhu X. Y.; Chen J. R.; Lu L. Q.; Xiao W. J. Tetrahedron 2012, 68, 6032. |
| [9] | Kondoh A.; Aoki T.; Terada M. Chem.-Eur. J. 2017, 23, 2769. |
| [10] | Li H. T.; Zhu Y. Q.; Lu D. F.; Gong Y. F. Org. Biomol. Chem. 2018, 16, 5907. |
| [11] | (a) Peng C.; Zhai J. J.; Xue M. Q.; Xu F. Org. Biomol. Chem. 2017, 15, 3968. |
| [11] | (b) Sun W. X.; Peng C.; Yao Z. G.; Xu F. Org. Biomol. Chem. 2019, 17, 6620. |
| [11] | (c) Chen Q. F.; Teng Y.; Xu F. Org. Lett. 2021, 23, 4785. |
| [12] | (a) Delhaye L.; Merschaert A.; Delbeke P.; Brione W. Org. Process Res. Dev. 2007, 11, 689. |
| [12] | (b) Bray C. D.; Faveri G. J. Org. Chem. 2010, 75, 4652. |
| [12] | (c) Kumar P.; Dubey A.; Harbindu A. Org. Biomol. Chem. 2012, 10, 6987. |
| [12] | (d) Sokolsky A.; Smith III A. B. Org. Lett. 2012, 14, 4470. |
| [13] | (a) Li K.; Lv Y.; Lu Z.; Yun X.; Yan S. Green Synth. Catal. 2022, 3, 59. |
| [13] | (b) Song Y.; Wang L.; Duan Z.; Mathey F. Chin. Chem. Lett. 2020, 31, 329. |
| [13] | (c) Fang C.; Wei B.; Ma D. Chin. J. Chem. 2021, 39, 2957. |
| [14] | (a) Zhou S.; Wang S.; Yang G.; Liu X.; Sheng E.; Zhang K.; Cheng L.; Huang Z. Polyhedron 2003, 22, 1019. |
| [14] | (b) Xie M.; Liu X.; Wang S.; Liu L.; Wu Y.; Yang G.; Zhou S.; Sheng E.; Huang Z. Chin. J. Chem. 2004, 22, 678. |
| [14] | (c) Sheng E.; Wang S.; Yang G.; Zhou S.; Cheng L.; Zhang K.; Huang Z. Organometallics 2003, 22, 684. |
| [15] | Lu H. H.; Wang X. F.; Yao C. J.; Zhang J. M.; Wu H.; Xiao W. J. Chem. Commun. 2009, 4251. |
/
| 〈 |
|
〉 |